Amid the coronavirus pandemic, pioneering pharmaceutical makers, research institutes, and universities are working in collaboration to develop novel antiviral drugs that are small compounds and vaccines. Although most inventions produced this year will be disclosed when their patent applications are published next year, the inventions being developed before this pandemic have already been disclosed.
In this article, we introduce promising antiviral drugs that are small compounds and vaccines for COVID-19, and analyse the relevant patents.
Antiviral drugs
Avigan® (Favipiravir)
[chemical structure]
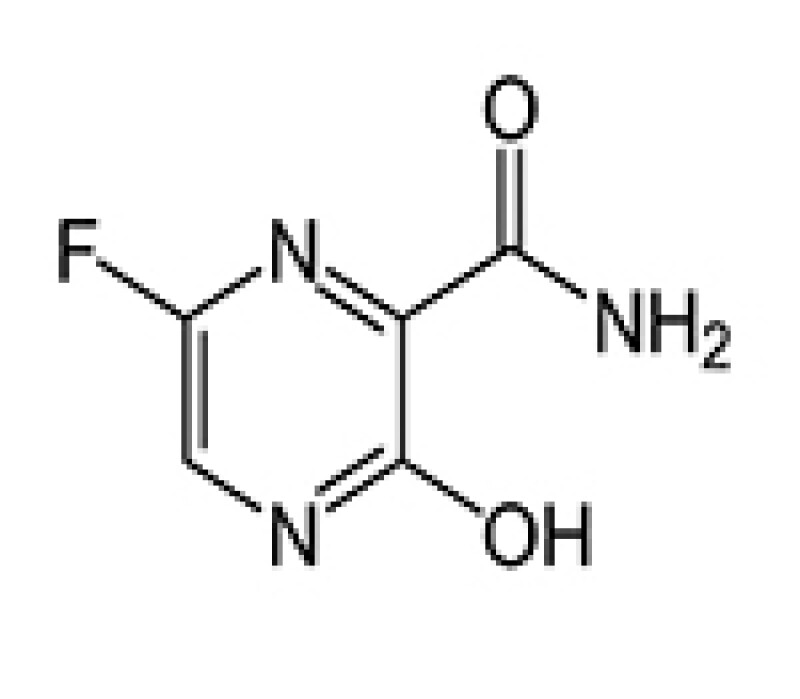
Avigan is a small molecule which is a viral ribonucleic acid (RNA)-dependent RNA polymerase inhibitor and is used in the treatment of influenza. Avigan was invented and patented by Fujifilm Toyama Chemical in Japan. Fujifilm has announced that the company is going to apply for sales and market approval to the Health, Labour and Welfare Ministry (HLWM) in Japan in October 2020. They have confirmed a positive outcome from their clinical trials after controversies regarding its efficacy. It will become the first approved domestic drug for COVID-19 once the government approves it, presumably in early December.
Patent information
Avigan is protected by both a manufacturing process patent and a substance patent. However, it is possible to manufacture Avigan without the following patented manufacturing process. In contrast, it should be noted that the substance patent expired on August 18 2019, but its patent term extension (PTE) registration will expire on November 11 2031. Since the extended right has only been applied for in relation to the treatment of influenza, this right does not cover the medical treatment of COVID-19.
1. Manufacturing process patent
Japanese patent number: 5787727
Patentee: Fujifilm Corporation, Toyama Chemical
Title of invention: The pyrazino [2,3 - d] isoxazole derivative
Expiration date: November 11 2031
2. Substance patent
Japanese patent number: 3453362
Patentee: Toyama Chemical
Title of invention: Nitrogenous heterocyclic carboxamide derivatives or salts thereof and antiviral agents containing both
Expiration date: August 18 2024
Claim interpretation of substance patent - Japanese patent number 3453362
Claim 1
An antiviral agent comprising a nitrogen-containing heterocyclic
carboxamide derivative represented by the following general formula:
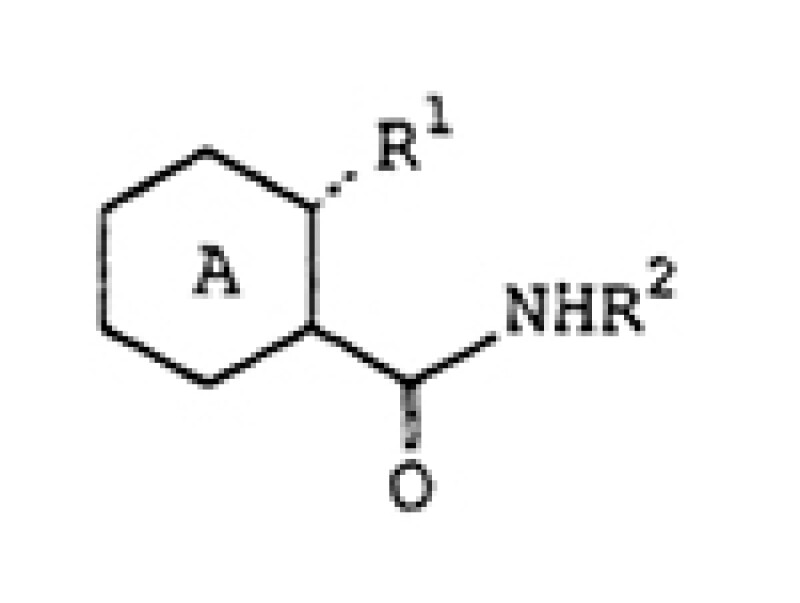
wherein ring A represents a substituted or unsubstituted pyrazine, pyrimidine, pyridazine or triazine ring; R1 represents..OH; R2 represents a hydrogen atom,..; and the broken line represents a single bond..; or a salt thereof.
Claim 1 is an agent claim, which specifies the use thereof, but the claimed scope is broad because the scope of ring A is broad.
Claim 6
A nitrogen-containing heterocyclic derivative represented by the following general formula:
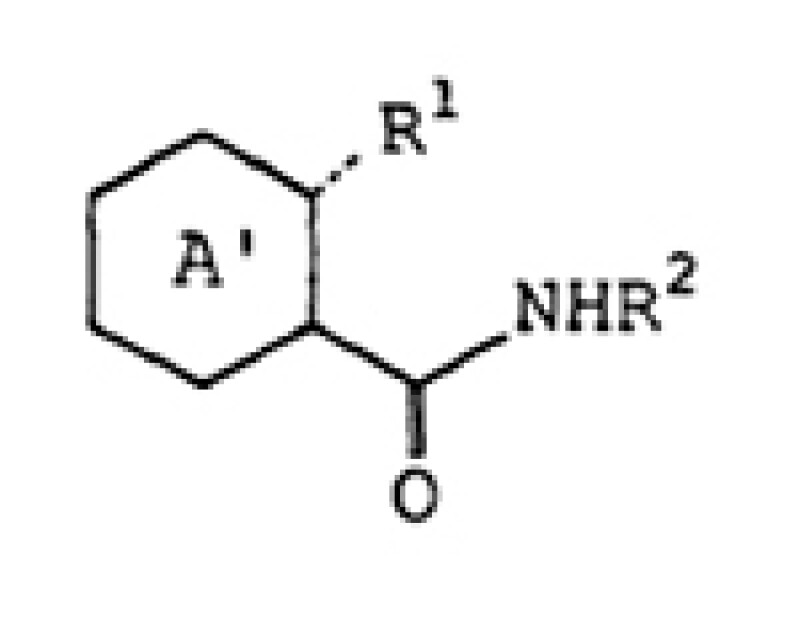
wherein ring A’ represents a pyrazine ring substituted with a halogen atom,.. ; R1 represents..OH; R2 represents a hydrogen atom..; and the broken line represents a single bond..; or a salt thereof.
Claim 6 is a compound claim which does not specify any usage.
Noteworthy point
While it takes a long time for clinical trials and pharmaceutical applications to be submitted to the Health, Labour and Welfare Ministry (HLWM), a patent on a drug can have a significant impact on both IP and society as a whole. Some patents come to draw a lot of attention when their rights are about to expire or have expired. We hope that this noble drug will help eradicate COVID-19.
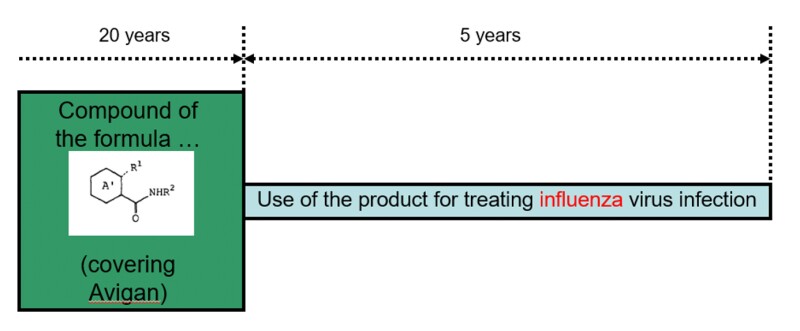
Patent term extension in Japan
The substance patent referred to above (Patent No.3453362) is still valid, but its use for the extension was limited to treatment for influenza (cf. Fig.1). As a result, the patentee cannot use this right commercially for COVID-19-related treatments.
Under Japanese Patent Law, the patent term is 20 years from the filing date. However, a patent term extension (PTE) system can be utilised for inventions relating to medicines and agricultural chemicals. This is a unique system in Japan which, as an exception, allows a patent right to be maintained even after the expiration of the duration of the patent right 20 years after the filing of the application by taking into account the time required to obtain sales and marketing approval for medicines. The extended period can be up to five years.
[Fig.1]
Veklury® (Remdesivir)
Remdesivir is a small molecule which is a pro-drug that is converted in the body into a ribonucleotide analogue and is used for treatment of the Ebola virus disease.
[chemical structure]
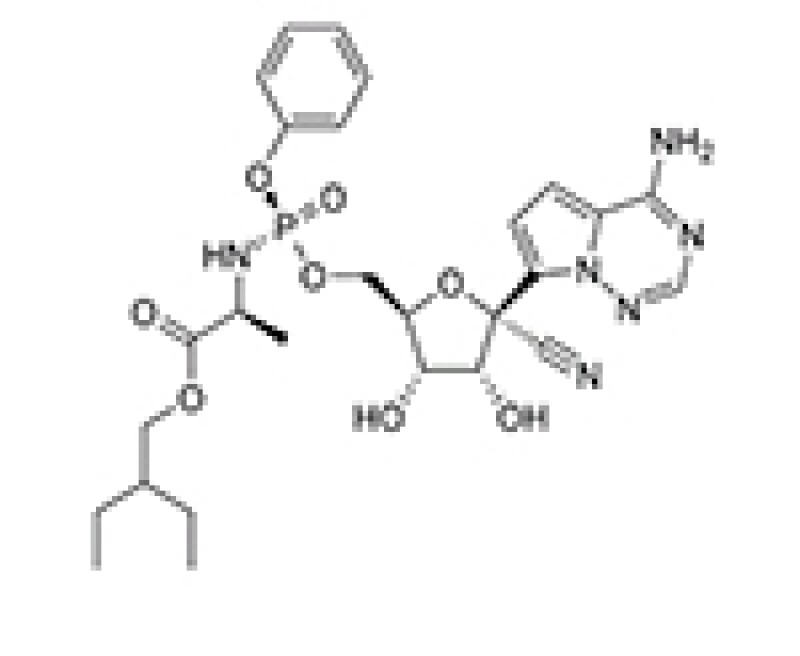
Patent information
Remdesivir is protected by a substance patent which is still pending not only in Japan but also in other countries.
Japanese Patent number: 5969471
Patentee: Gilead Sciences
Title of invention: Methods and compounds for treating paramyxoviridae virus infections
Expiration date: July 22 2031
Claim interpretation
Claim 23
The compound of claim 20 that is
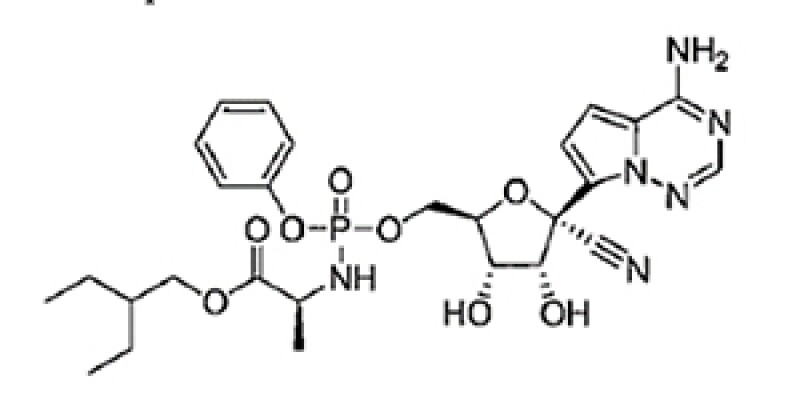
or a pharmaceutically acceptable salt thereof.
Claim 23 is a compound claim which does not specify any usage.
Amended Claim 36
A composition for treating a coronaviridae infection in a human in need thereof, comprising a compound having the following structure
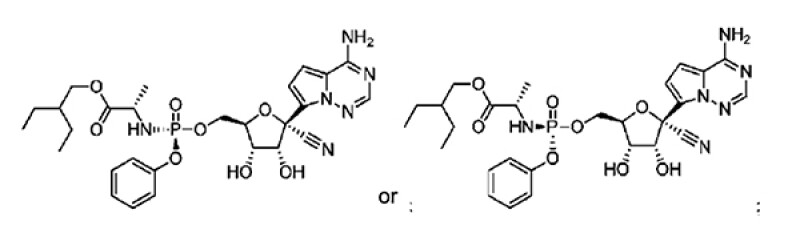
or a pharmaceutically acceptable salt thereof.
Claim 36 is a composition claim for treating a coronaviridae infection.
In the example, MERS-CoV and SARS-CoV antiviral activity are shown.
Comparison between Avigan and Remdesivir
Efficacy
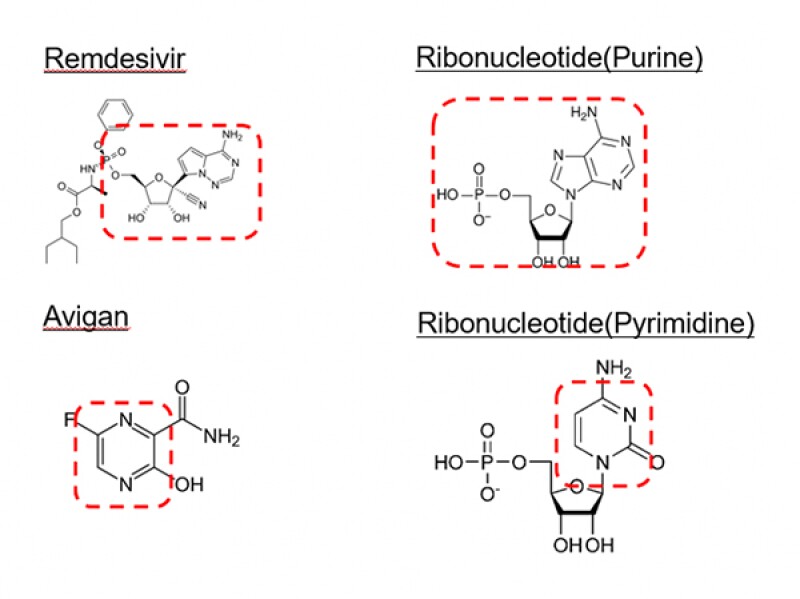
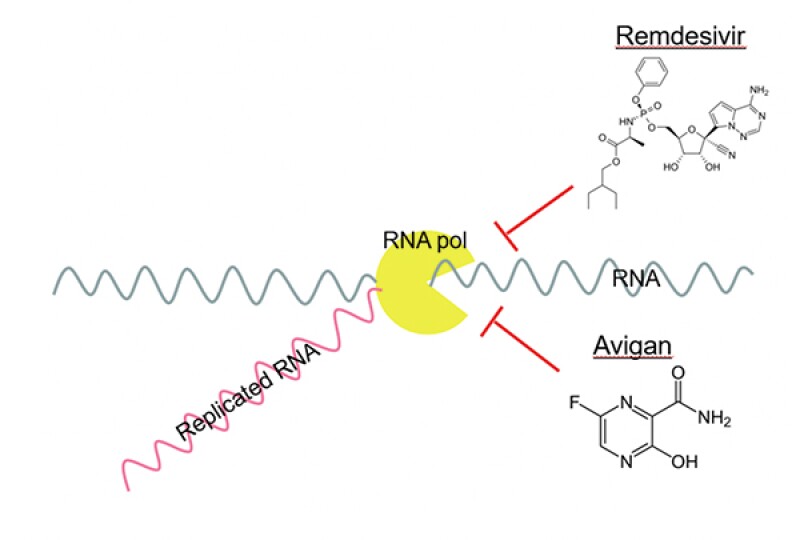
Both compounds have a similar structure as a nucleotide (cf. Fig.2) and function as nucleotide analogues which inhibit the replication of RNA by RNA polymerase in a competitive manner (cf. Fig.3).
[Fig.2]
[Fig.3]
Patent
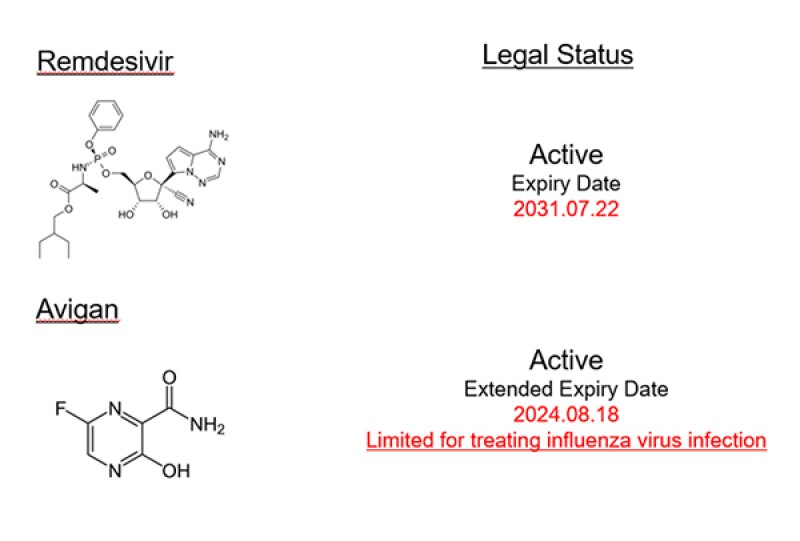
Regarding the legal status of both compounds, Remdesivir is protected by its patent while Avigan’s patent, which protects the treatment of COVID-19, has expired (cf. Fig.4).
[Fig.4]
Vaccines
The World Health Organization (WHO) has announced that 35 vaccines are now under clinical trial and another 145 are in a pre-clinical evaluation stage (as of September 9 2020). Although most of the promising vaccine candidates in the WHO list have not yet been disclosed by their patent publication, pharmaceutical companies are likely to tap their existing technologies and owned patents so as to apply them to coronavirus vaccine development. We have focused on two promising COVID-19 vaccines under clinical trial from data in the DRAFT landscape of COVID-19 candidate vaccines - 9 September 2020.
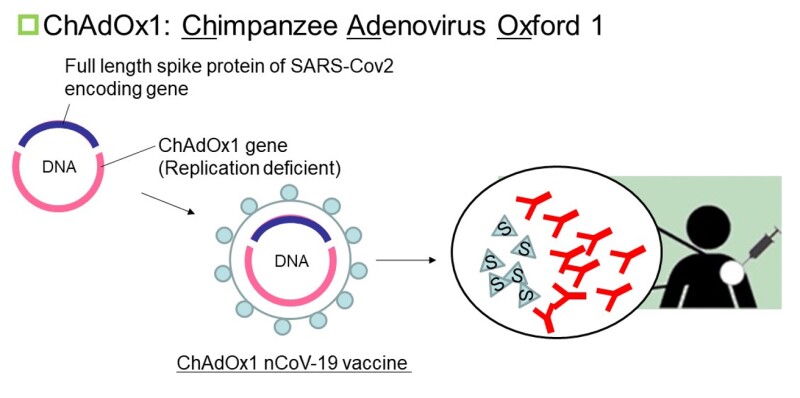
1. ChAdOx1-S (AZD1222) (developer/manufacturer: University of Oxford/AstraZeneca)
This vaccine is a non-replicating viral vector. A viral vector vaccine is a non-pathogenic or a weakened viral vector where antigen protein genes are genetically introduced into the vector. Adenovirus and retrovirus are used as viral vectors. For the SARS-CoV-2 vaccine, a viral vector vaccine having a gene encoding a spike protein, which plays a crucial role in infection, is mainly being developed (cf. Fig.5).
Patent information
Patent number: 6230527
Patentee: University of Oxford
Title of invention: Simian adenovirus and hybrid adenoviral vectors
Claim interpretation
Claim 1
An adenovirus vector comprising a capsid, wherein the capsid contains one or more capsid protein derived from wild type chimpanzee adenovirus AdY25, and encapsidates a nucleic acid molecule comprising an exogeneous nucleotide sequence of interest operably linked to expression control sequences which direct the translation, transcription and/or expression thereof in an animal cell and an adenoviral packaging signal sequence, wherein nucleotide sequence coding the wild type chimpanzee adenovirus AdY25 is sequence number 1.
Noteworthy point
In the present invention, the prevalence rate of the vector-neutralising antibodies in human serum is low, and the vector, which has a target antigen, induces a sufficiently high immune system. There is no limitation in the claims to the antigen of SARS-CoV-2. Any adenovirus vector comprising one or more capsid derived from wild type chimpanzee adenovirus AdY25 would be within the claim scope. Since the present invention does not specify a target antigen, the invention could not stand out from competitors if the aforementioned vector is of no use.
[Fig.5]
2. mRNA-1273 (developer/manufacturer: Moderna/NIAID)
This vaccine is an LNP-encapsulated mRNA. The mRNA vaccine for SARS-CoV-2 is engineered with the following steps: first, an artificial mRNA strand encoding a coronavirus gene is produced. Second, the mRNA is encapsuled by lipids, which are nanoparticles. Third, once the vaccine is injected into a human body, mRNAs are translated into virus proteins (virus antigen) and then, they cause the immune system to react which subsequently creates antibodies. Compared with conventional vaccines, mRNA vaccines are available in a shorter manufacturing time and are less cumbersome in culturing the virus. In recent years, there has been a lot of activity in mRNA vaccine development.
Patent information
US Patent Registration number: 10702600 (Registration date: July 7 2020)
Patentee: Moderna
Title of Invention: Betacoronavirus mRNA vaccine
Claim interpretation
Currently, there is no corresponding Japanese application. Below is an excerpt from the US patent gazette.
Claim 1
A composition, comprising a messenger ribonucleic acid (mRNA) comprising an open reading frame encoding a betacoronavirus (BetaCoV) S protein or S protein subunit formulated in a lipid nanoparticle.
Noteworthy point
BetaCoV recited in Claim 1 covers novel coronavirus (SARS-CoV2). The composition including mRNA encoding coronavirus S proteins within liquid nanoparticles, is covered in a scope of claims in the present invention. An example of the present invention describes the result that the mRNA vaccine coding MERS CoV spike protein had on immunised rabbits, successfully inducing a neutralising antibody.
Some antiviral drugs that are being developed for clinical trials are protected by their existing patents, but these protections are due to a broad claim scope or related patents such as vectors. Holding a core patent at an earlier stage is essential for helping to boost further drug discovery in this unprecedented situation.
Furthermore, patent applications directly protect antiviral drugs that are under development and will be disclosed in the near future.












