The patent grant rate in Japan increased to 82% in 2021 from 56% in 2009 since the Intellectual Property High Court relaxed the inventive step hurdle in 2009. However, sometimes the inventive step hurdle is severe in biotech and pharmaceutical inventions.
Even though an invention has different features or structures from the prior art, an invention that is easy to obtain by known methods and/or that does not have an unpredictable significant effect will be denied in terms of inventive step. To overcome an inventive step rejection, applicants should assert the difficulty of the invention to obtain the product and unpredictable advantageous effects when they prepare patent applications.
Combination therapy, or combo therapy, involves the combination of two or more drugs to treat a disease. Significant efficacy is sometimes observed by the combination of known drugs. In such a case, the combination of known drugs is filed in an application as a new invention. Whether the combination of known drugs can jump over the inventive step hurdle is a key issue to be resolved.
This article discusses inventive steps in focusing on combo therapy inventions and suggests the best way to jump over the inventive step hurdles in biotech and pharmaceutical inventions.
The JPO’s inventive step guidance
The JPO provides examples in which an inventive step is denied or affirmed. The below scenarios are introduced in the JPO’s Examination Handbook for Patents and Utility Models.
Inventive step is denied in a combo therapy invention in the following cases:
A combination of known components with the same primary action; for example, a liquid anti-flatulent agent comprising dietary fibre (a known anti-flatulent agent) and bacteria X (a known anti-flatulent agent);
A combination of a known primary component and a known secondary component that can solve the problem of the primary component; for example, a therapeutic agent for paclitaxel-responsive tumours comprising a combination of paclitaxel, which has vomiting as a known side effect, and compound X, which has a known anti-vomiting effect; and
A combination of components known to have a therapeutic effect on each symptom arising from the primary disease; for example, an AIDS therapeutic agent comprising a combination of azidothymidine, a known anti-HIV drug, and compound X, a known pneumonia drug, with it being well known that AIDS causes pneumonia.
The following is an example where inventive step is affirmed in a combo therapy invention: a combination of known components that shows a remarkable effect beyond prediction; e.g., a composition to treat diabetes comprising compound A (a known anti-diabetic drug) and compound B (a known anti-diabetic drug) in a ratio of 5:1 to 4:1 by weight. The combination of compound A and compound B in a specific ratio was found to provide a reduction of side effects, such as weight gain, which were observed with the use of compound A alone.
The following case studies take a closer look at claims related to combo therapy inventions.
Case study one
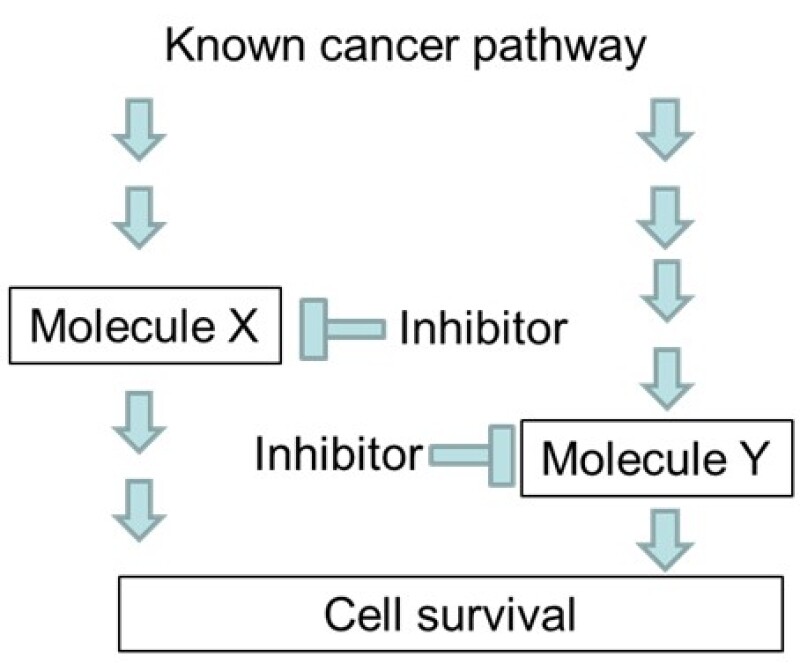
The applicant found that the disruption of known cancer pathways mediated by molecule X and molecule Y caused a significant anti-tumour effect (Figure 1) and then filed an application with the claim.
Claim – an agent for treating a cancer comprising a combination of a molecule X inhibitor and a molecule Y inhibitor.
Prior art – the examiner found prior art (Figure 2) and issued an inventive step rejection, as shown (Citation A, administration of a molecule X inhibitor to a cancer patient, and Citation B, administration of a molecule Y inhibitor to a cancer patient).
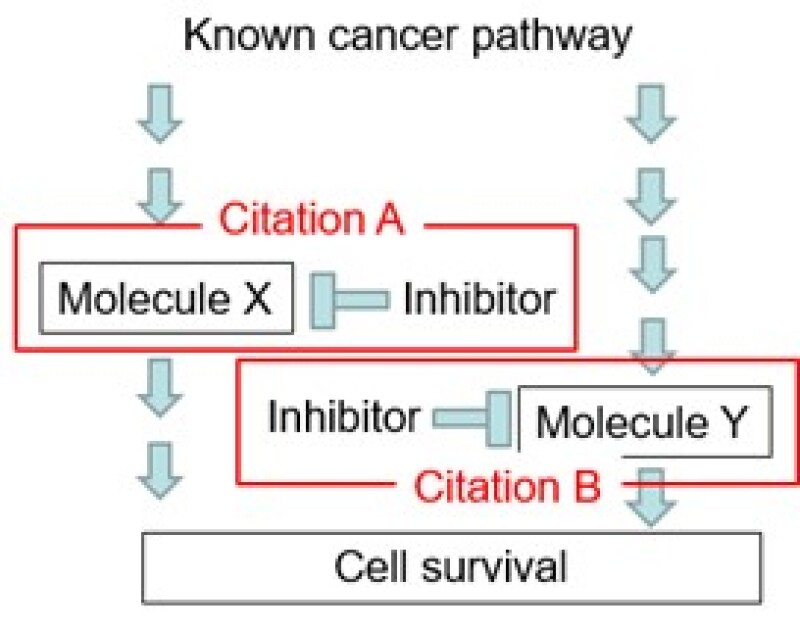
Inventive step rejection – optimising a combination of two or more pharmaceutical ingredients to solve problems well known to those skilled in the art, such as increasing drug efficacy, is a normal exercise of creative ability for those skilled in the art.
If we look at the present specification, the combination of compounds A and B showed a synergistic effect compared to compound A or B alone (Figure 3).
Amended claim – based on the example in the present specification (Figure 3), the claim can be amended as shown (Figure 4), involving an agent for treating a cancer comprising a combination of compound A and compound B.
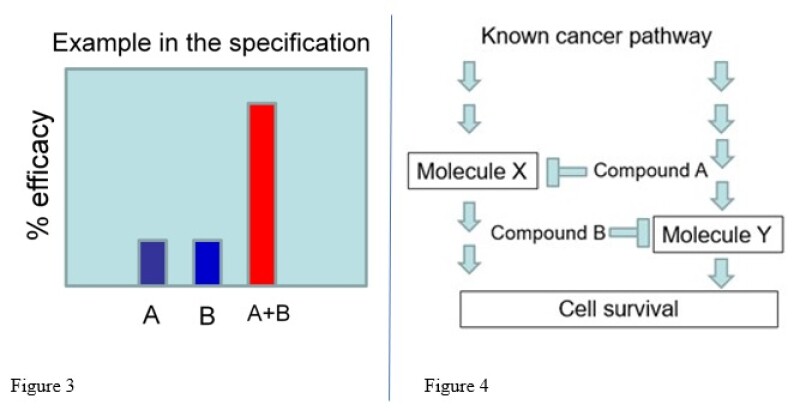
In the remarks, it can be asserted that the advantageous effect caused by the combination of compounds A and B is beyond prediction based on citations A and B.
Case study two
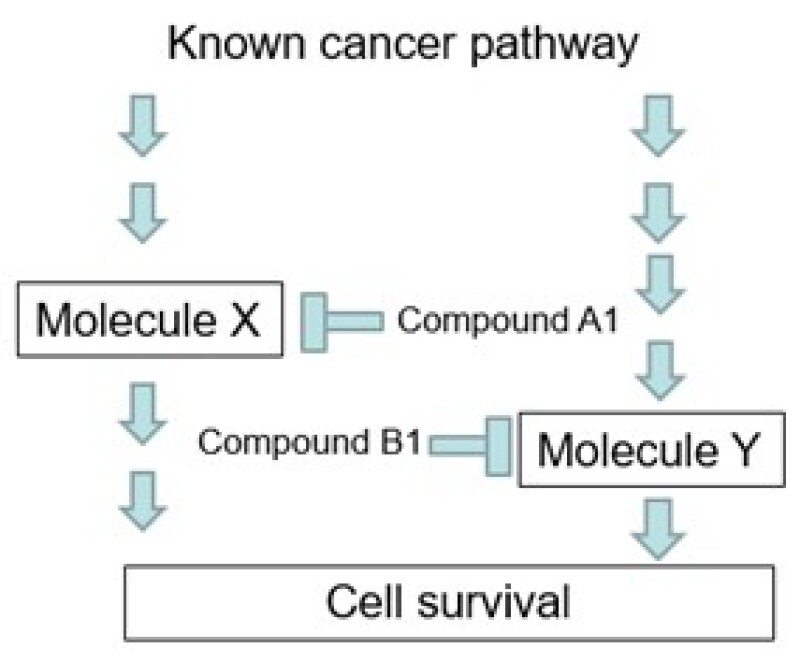
The applicant found that the combination of compound A1 and compound B1 caused a significant anti-tumour effect in lung cancer (Figure 5) and then filed an application with the claim, as shown. As background, compound A1 is known as a molecule X inhibitor and compound B1 is known as a molecule Y inhibitor.
Claim – an agent for treating lung cancer comprising a combination of compound A1 and compound B1.
Prior art – the examiner found prior art (Figure 6) and issued an inventive step rejection, as shown.
Citation A:
Claim – a method for treating lung cancer, comprising administering an effective amount of the molecule X inhibitor and the molecule Y inhibitor to a cancer patient.
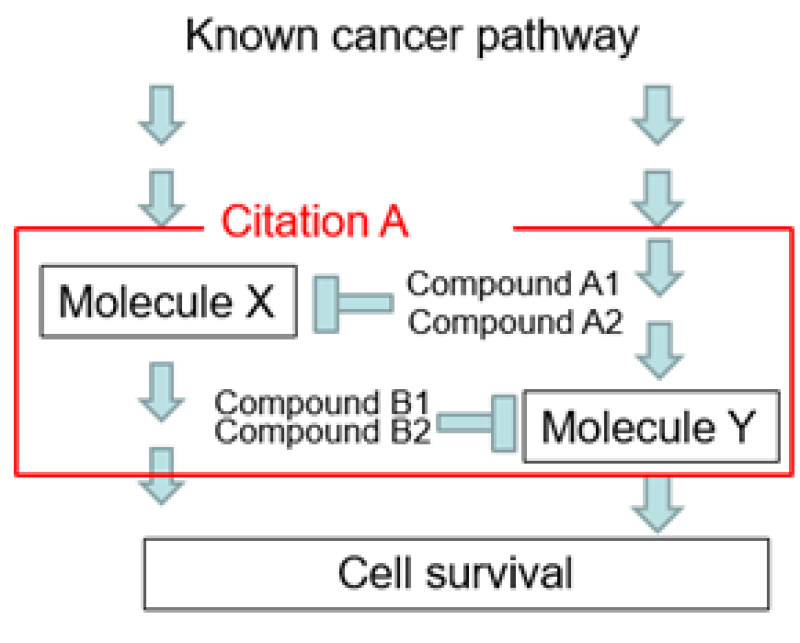
Specification – the molecule X inhibitor includes A1, A2, etc, while the molecule Y inhibitor includes B1, B2, etc.
Example – combination of A2 and B2 in an animal lung cancer model.
Inventive step rejection – since the therapeutic effect on lung cancer by the combination of the X inhibitor and the Y inhibitor is shown in the examples of the citation, combined use of compounds A1 and B1 can be easily conceived by those skilled in the art.
While Citation A is a genus invention, the present invention is a species invention. This hurdle seems to be very high. To tackle this rejection, there are several points to be considered:
We should look for an advantageous effect of A1 and B1 (efficacy, side effect, etc.) in the examples of the present specification. Comparison data (A1+B1>A2+B2) would be better to assert the advantageous effect.
We should look for a specific feature, such as dosing regimen or type of cancer, in the specification for amendment.
We should look for obstructive factors to combine A1 and B1 from the citation. If the citation discloses that A1 diminishes the efficacy of B1, this disclosure is an appropriate obstructive factor.
We should consider no motivation to combine A1 and B1 from the citation. For example, we can assert that the citation has very long lists of the molecule X inhibitor and the molecule Y inhibitor, and it does not specifically disclose combining A1 and B1.
We should consider later submission of experimental data. Precisely, we should consider submitting experimental data to show the advantageous effect of A1 and B1 (efficacy, side effect, etc.). In particular, comparison data (A1+B1>A2+B2) would be better.
Later submission of experimental data
According to the JPO Examination Guidelines for Patents and Utility Models, advantageous effects in later submission of experimental data shall be taken into consideration in the following cases:
The effect is disclosed in the specification; or
The effect is not disclosed in the specification but can be inferred from the specification by a person skilled in the art.
Therefore, based on a partial description of the effect in the specification, later submission of data would be effective.
In the case that obtaining new experimental data is difficult because time has passed after the original filing, clinical trial data can be utilised, such as presentation material in an international conference. In addition, in the event that a client still would not have any documents, searches should be conducted in databases (for example, PubMed) for scientific papers that the inventor published.
Reducing the burden in patenting a pharmaceutical invention
When preparing a patent application for a pharmaceutical invention, challenging inventive step is key to patent obtainment. Learning the above practice is one way for an applicant to reduce their burden in patent obtainment.












