Effective searching is critical for protecting intellectual property (IP) and building stronger patent programmes in biologics R&D. However, the intricate nature of biologics innovation and IP – including sequence data, chemical modifications, and novel formulations – can make comprehensive searches challenging. This article presents several tips for securing biologics IP to safeguard your innovations, navigate regulatory frameworks, and mitigate potential litigation risks.
An opportunity and a challenge
Biologics IP has grown exponentially, creating opportunities for pharmaceutical companies to drive business value by developing stronger patent programmes at every stage of R&D.
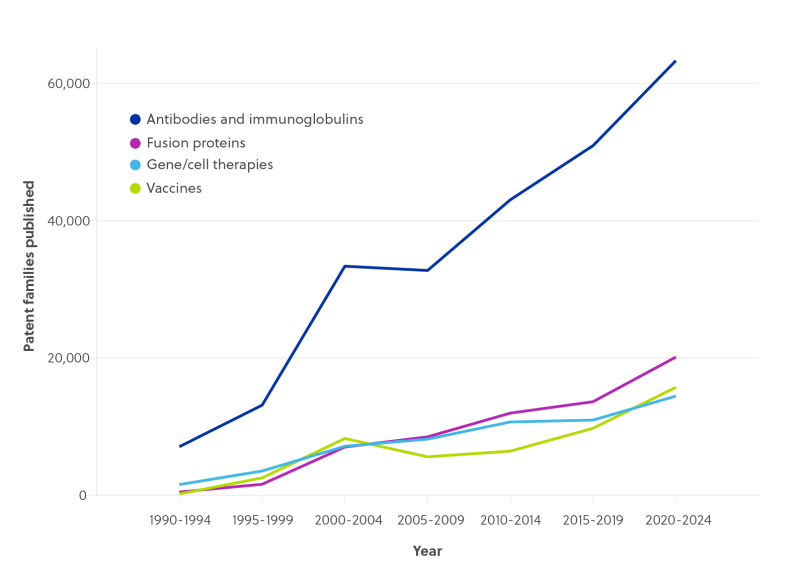
However, this growing volume of data adds another layer of complexity to an already challenging task. Though reliable sequence searches are crucial for pinpointing the business value of innovations during R&D and in building patent programmes to protect them, information on sequences is difficult to search and retrieve.
Pharmaceutical companies can encounter a number of obstacles that prevent efficient information access, including:
Sequence database records lacking essential information about chemical modifications;
Sequences claimed in different ways within patents;
Inconsistent descriptions of sequences in scientific and patent literature over time; and
Sequences as one component of complex inventions (such as antibody-drug conjugates with the antibody, drug payload, and linker).
These issues can lead to incomplete or inaccurate search results, which may hinder your ability to secure robust patent claims.
Foster collaboration across R&D, IP, and search
The complexity of biologics IP requires seamless collaboration between R&D, IP teams, outside counsel, and search professionals. Expectations, goals, and responsibilities should be aligned across all teams to reduce errors and ensure comprehensive search results. Searches should be treated as dynamic processes – results must be reviewed and refined as new data emerges during R&D, with each team member actively verifying scientific and patent data to minimise the risk of oversight.
Define your goals for strategic searching
When planning a search, focus not only on what you want to search for but also on the end goal. Approach the process with the question: “What would I be concerned about in a disclosed patent claim?” Sequence searches are inherently complex and demand more than traditional approaches:
Basic BLAST (basic local alignment search tool) or motif searches may not suffice, and queries often need to cover sequences, chemical modifications, keywords, and structures.
Skilled searchers leverage a variety of databases and platforms to create tailored strategies that efficiently handle large numbers of sequences.
The search process is iterative, evolving as results are reviewed. For instance, an initial request for a linker with five repeatable units may shift to evaluating linkers with fewer units to uncover nearby patent issues.
This flexible mindset ensures you capture a broader range of potential conflicts and comprehensive biologics IP insights that support informed decision making.
Leverage advanced analytical tools
Traditional methods of analysing search results can be highly manual, time consuming, and prone to human error, requiring searchers to spend days combing through citations and assembling reports. Data analytics platforms and analytical methods such as variation analysis can be used to interpret search results, significantly streamlining analysis and packaging data into reporting formats that can be easily filtered, reviewed, and understood.
Use freedom-to-operate searches throughout R&D
Think of your patent programme as an onion: a core composition of matter patent filing surrounded by protective patent filings for innovations that arise during R&D. Considering IP insights early and throughout R&D guides research, supports strong claims, and provides new layers of protection at every stage.
Lead development – Freedom-to-operate (FTO) searches can be performed with wide variability around the lead candidates to identify likely patent threats;
Proof of concept – FTO searches are used to clear core innovations and establish patentability, particularly when first-to-file status is critical for attracting investors; and
After phase two trials – additional FTO searches can be performed to clear novel uses, formulations, processes, and other innovations.
Continuously monitor the patent landscape
New patent applications are not published for 18 months, making continuous monitoring essential to staying ahead of potential threats. Your monitoring programme should alert you to:
Newly published patent applications that may affect ongoing R&D;
Scientific publications relevant to your work; and
Changes in the legal status or prosecution of patents.
This proactive approach ensures your team remains informed and prepared to adapt to emerging risks throughout the R&D process.
The complexities inherent in biologics require a sophisticated approach to IP searches. By adopting strategic search methodologies, leveraging specialised tools, and incorporating advanced analytics, organisations can build a stronger foundation for innovation protection. CAS IP Services works with leading biologics innovators to help to streamline their approach to patent monitoring and IP management. By collaborating with the CAS team, you can utilise its expertise in biologics IP to build a stronger patent programme and secure your future innovations.











