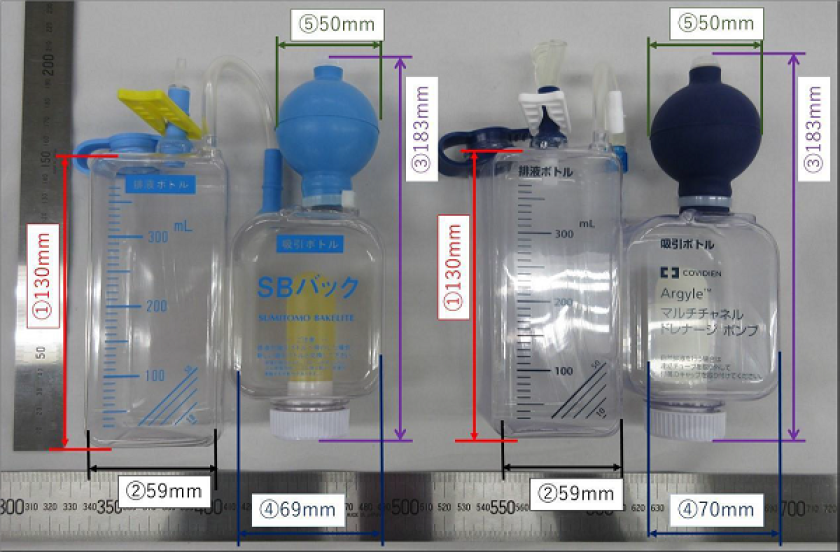
Summary of the case
Sumitomo Bakelite has manufactured and sold each device comprising a portable and disposable device for continuous low pressure suction or a set of these devices (collectively SB Bag) with the product name "SB Bag" since 1984. Those consisting of a drainage bottle and a suction bottle in SB Bag are the relevant products belonging to Sumitomo Bakelite in this case. Nippon Covidien has manufactured and sold its product since January 2018.
Sumitomo Bakelite brought a case against Nippon Covidien stating that the manufacture and sale of Nippon Covidien's product, having a configuration similar to that of Sumitomo Bakelite's product, which is well-known among consumers as Sumitomo Bakelite's indication of goods or business, is an act that creates confusion with Sumitomo Bakelite's product. It claimed that it falls under an act of unfair competition as stipulated in Article 2(1)(i) of the Unfair Competition Prevention Act (act) and demanded an injunction against the manufacture of Nippon Covidien's product.
Judgment of December 26 2018, Tokyo District Court
The Tokyo District Court (Presiding Judge Yamada) determined that while it is acknowledged that the configuration of Sumitomo Bakelite's product is well-known among consumers as Sumitomo Bakelite's indication of goods or business, and that the configuration of Nippon Covidien's product is similar to that of Sumitomo Bakelite's product, it cannot be acknowledged that the act by Nippon Covidien of manufacturing and selling Nippon Covidien's product falls under an "act that creates confusion" with Sumitomo Bakelite's product. It thus dismissed Sumitomo Bakelite's claims entirely.
When a medical institution newly purchases a medical device, it is common practice for healthcare professionals to receive an explanation of the medical device, including its characteristics, functions and method of use, from a sales representative of a manufacturer of medical devices or a distributor. The institution can then make a decision on whether to adopt the medical device and place an order for the same to a distributor of medical devices. Moreover, at many medical institutions, there is a rule known as “buy-one-to-replace-another", according to which, concerning medical devices of the same type, only one type of such items shall be adopted.
Sumitomo Bakelite's product and Nippon Covidien's product have their own product name and company name indicated on the product itself, different catalogues are created for each product and sold separately, and the healthcare professionals, who are consumers, also have expertise in medical equipment. Therefore it was not accepted that, in the sale of Nippon Covidien's product, there is a likelihood of misleading healthcare professionals, who are consumers, that the source or origin of the products is the same. It was not accepted that there is a likelihood of misleading consumers regarding any relationship between Sumitomo Bakelite and Nippon Covidien.
Judgment of August 29 2019, IP High Court
The IP High Court (Presiding Judge Otaka) affirmed the points relating to being well-known and similarity. It modified the Tokyo District Court judgment, upholding a part of Sumitomo Bakelite's claims.
The actual circumstances involved in the process of a transaction of a medical device are as follows:
[i] When a medical institution newly purchases a medical device, a common practice is that healthcare professionals receive an explanation about the medical device, including its characteristics, functions, and method of use, from a sales representative of a manufacturer of medical devices or a distributor at a product briefing, and then use the medical device experimentally for approximately one week to one month in clinical practice. After evaluating the medical device in terms of usefulness and function, they make a decision to adopt the medical device, and place an order for the same with a manufacturer of medical devices or with a distributor. At medical institutions whose number of hospital beds is constant, a materials committee consisting of physicians, nurses, etc. holds a meeting, and after discussions among committee members, the decision of whether to purchase the medical device is made. On the other hand, at private hospitals and medical institutions which have fewer hospital beds, no materials committee meeting is held, and it is not rare for the decision to adopt a medical device to be made by physicians.
[ii] When a medical institution purchases a medical device which has been used by the medical institution before, sometimes the purchase is made by referring to a catalogue for medical use containing information such as the image, product number, specifications, and price of each medical device, and an order is placed for the same, or the medical institution may purchase the same through an online shopping site.
[iii] In cases of relatively inexpensive medical devices such as consumables, a medical institution may purchase the same, even if it is the first time making such a purchase, by referring to a catalogue for medical use and informing the sales representative of a manufacturer of medical devices (or a distributor) of the product number and other information, or through an online shopping site.
[iv] At a medical institution, there is a rule known as “buy-one-to-replace-another," according to which, concerning medical devices that have the same use and similar capacities, only one type of such items shall be adopted, and introduction of a new medical device is conditional on the disposal of another medical device of the same type and effect. The "buy-one-to-replace-another” rule is adopted mostly at large-scale medical institutions such as university hospitals, but at small scale medical institutions, there is a strong tendency for each physician to use a medical device with which the physician is most familiar, so the "buy-one-to-replace-another” rule may not be adopted. Even at medical institutions where the "buy-one-to-replace-another” rule is adopted, the rule may not be thoroughly implemented, and specific doctors may designate and use different medical devices based on their treatment policies.
It was acknowledged that, as a result of continuous and exclusive use by Sumitomo Bakelite over a long period of approximately 34 years, the configuration of Sumitomo Bakelite's product came to acquire, among healthcare professionals who are consumers, a function as an indication of source or origin showing that the product comes from a specific business entity, in addition to being well-known as an indication of source or origin for Sumitomo Bakelite's product. Under such circumstances, Nippon Covidien began selling Nippon Covidien's product. Its configuration very closely resembles that of Sumitomo Bakelite's product. Furthermore, considering that both products are medical devices which are categorised under consumables and the products have a sales method in common, in cases where healthcare professionals come upon the configuration of Nippon Covidien's product, which very closely resembles Sumitomo Bakelite's product in configuration, through product images indicated in a catalogue for medical devices or on an online shopping site, there is a likelihood of misleading consumers into thinking that the source or origin of the products is the same. It was therefore acknowledged that the sale of Nippon Covidien's product by Nippon Covidien is an act which creates confusion with Sumitomo Bakelite's product.
Supreme Court
The Supreme Court denied certiorari to a petition for writ of certiorari filed by Nippon Covidien.
Practical tips
For an "act that creates confusion" to be established, it is sufficient if there is likelihood that confusion is created, and it is not necessary to actually cause confusion. If the requirements for being well-known and similarity are established, the likelihood of confusion may also be affirmed in principle, but if there are special circumstances, the likelihood of confusion may be denied. The Tokyo District Court affirmed the requirements for being well-known and similarity, but denied the likelihood of confusion, taking into consideration the actual circumstances of a medical device transaction, where healthcare professionals receive an explanation about the medical device from a sales representative of a manufacturer of medical devices or a distributor, and make a decision to adopt the medical device, and place an order for the same with a distributor of medical devices. In contrast, the IP High Court affirmed the likelihood of confusion. What is the reason for this difference?
Firstly, Sumitomo Bakelite, who lost in the first instance, carefully claimed and clarified with new evidence, the actual circumstances of the medical device transaction. These circumstances were the cause of the loss. They claimed and proved that Sumitomo Bakelite and Nippon Covidien's products are categorised under consumables and are also sold on online shopping sites in addition to catalogue sales. They also claimed and proved that the “buy-one-to-replace-another" rule, which the Tokyo District Court judgment seems to have relied upon heavily, is not an absolute rule and may not be adopted at small-scale medical institutions, or even if it is adopted, it may not be thoroughly implemented. As a result, the Tokyo District Court determined that there is no likelihood of misleading consumers that the source or origin of the products is the same by emphasising that healthcare professionals purchase the medical devices through careful interactions with manufacturers of medical devices etc. The IP High Court determined, based on Sumitomo Bakelite's new argument, that the medical device in this case is an inexpensive consumable, and it does not always go through such careful interactions. The product may be purchased based on the configuration of the product through product images indicated in a catalogue or on an online shopping site. It then concluded that there is a likelihood of misleading customers that the source or origin of the products is the same.
Another point may be the difference in the court's attitude towards an "act that creates confusion.” Sumitomo Bakelite, in the statement of reasons for appeal, criticised the Tokyo District Court for demanding that confusion is actually created. On the other hand, in the statement of reasons for petition for acceptance of final appeal, Nippon Covidien accused the IP High Court of affirming erroneously that "likelihood of confusion" with the existence of abstract risk, where healthcare professionals come upon the configuration of Nippon Covidien's product, which very closely resembles Sumitomo Bakelite's product in configuration, leads to likelihood of misleading customers that the source or origin of the products is the same. In this way, the difference of view regarding the "likelihood of confusion" between the Tokyo District Court, which asserted that the existence of a concrete risk is necessary, and the IP High Court, which stated that the existence of an abstract risk is sufficient, led to the courts issuing differing judgments.