As we have seen with diagnostic robots equipped with artificial intelligence (AI), tailor-made medicines, and so forth, technical innovation has accelerated far beyond conventional technologies. Companies have come to place more importance on IP enforcement than ever as part of their corporate management strategies in order to cope with fierce global competition. In addition, the importance of design rights is growing in the business world. In April 2019, the Japan Patent Office (JPO) revised Japanese IP-related laws in response to the tide of emerging technologies and the necessity of establishing an environment which is favourable to applicants and right holders. This article will introduce and analyse recent developments in Japanese IP legislation which will come into force between 2018 and 2020.
Patents
AI-related inventions
Novel technologies regarding AI-related inventions have emerged in a wide range of technical fields from electronics to cutting-edge medical treatments and mechanical engineering etc. The newly revised JPO Examination Handbook, which was published in January 2019, illustrates concrete examples of judgements on description requirements, including enablement requirements and the inventive step of AI-related inventions. This revised handbook makes it easier for applicants to predict the JPO examiners' way of thinking and hence, makes it easier for them to draft and prosecute their patent applications more efficiently. The following shows the summary of points in the revised JPO Examination Handbook.
Description requirements
Whether enablement requirements are satisfied or not is determined by the existence of a correlation between input/output parameters of training data. The disclosure of a concrete correlation does not need to be written in the detailed description of an invention. However, in a case where a correlation cannot be presumed in light of common technical knowledge at the time of filing, the enablement requirements may not be satisfied. In addition, the invention of a product which is presumed to have a certain characteristic by using a trained model, is deemed to satisfy the enablement requirements if either of the following conditions are fulfilled:
a functional evaluation of an actual product is written in the embodiments; or
the verification of accuracy of a trained model is described in a working example.
Inventive step
The systematisation of human operations with artificial intelligence and the modification of machine learning methods may be deemed as lacking inventive step. On the other hand, in cases where the addition of training data used in machine learning methods has a remarkable effect, or the pre-processing of training data used in machine learning methods includes technical features which overcome inventive step, the claimed invention in question is considered to have inventive step.
Antibody drugs
Antibody drugs are known as promising medicines for curing refractory diseases, and are drawing global attention. When filing a patent application relating to antibody drugs, whether the application satisfies the support requirement is one thing that needs to be considered in particular. In other words, an applicant has to be aware of the following: to what extent should working examples and comparative examples be disclosed? Generally, medical-related inventions include complicated structures of low-molecular compounds and proteins. The structure-activity relationship, the so-called "SAR" must meet the support requirements. Therefore, applicants need to describe as many working examples as possible to satisfy the support requirement. At first glance, applicants tend to think that disclosing many working examples may help enlarge the scope of the claims. However, they should be aware that massive data disclosure, which is irrelevant to drug efficacy, may pose a risk of narrowing the scope of the claims.
For example, Optivo, also known as Nivolumab, is used mainly as a therapeutic medicine against non-small cell cancers and kidney cell cancer. Optivo is PD-1 (programmed cell death-1) monochronal antibody. The relevant patents are Japanese Patent (JP) Nos. 4,409,430; 5,159,730; and 6,035,372. JP No. 4,409,430 is the parent application of JP No. 5,159,730, and JP No. 6,035,372. The scope of protection described in Claim 1 of the aforementioned three patents is very broad. This means that the scope of protection in Claim 1 covers a malignant melanoma therapeutic agent whose active ingredients include a PD-1(programmed cell death-1) monochronal antibody, regardless of the amino acid sequence of heavy chains and light chains which comprise a PD-1monochronal antibody.
The earlier three patents were considered to be patentable when applying the anti-PD-1 antibody to an anti-cancer agent. In the working examples that support Claim 1 of the above three patents, the xenograft model is described as using only a single PD-1 monoclonal antibody that has an anti-metastatic effect. At the same time, a cancer cell transplant experiment using PD-1 knockout mice has revealed data indicating that it inhibits the proliferation of cancer cells.
However, as has been controversial in other antibody drug-related patents, there is room for further discussion in the above case as to what extent working examples should be disclosed, namely, how many examples of monoclonal antibody types should be given when disclosing the drug efficacy data.
Litigation
New criteria for calculating compensation for damages
The current patent law does not allow a patentee to seek compensation for damages that exceed a patentee's production capability and sales. After the revision, a patentee will be able to seek compensation for damages exceeding the patentee's production capability and sales in the form of an amount equivalent to a licensing fee. In addition, it is stated in the law that the amount based on a licensing fee shall be determined under an assumption that patent infringement was found.
Designs
The revision to the Design Law will come into effect in 2020 with the aim of a broader protection of designs utilising digital technologies, using design rights for brand-building, and realisation of a user-friendly filing system. The outline of the revision is as follows:
Graphic User Interfaces (GUIs)
Under the current practice, a GUI is protected as a part of the article on which the GUI is stored, but a GUI itself, such as GUIs of a web application, is exempted from the scope of protection. The revision allows GUIs to be protected independently of objects. After the revision, for example, GUIs which are stored on the cloud and provided through a network will be protectable.
Exterior and interior designs of buildings
The exterior and interior designs of buildings will be protectable subject matter under the Design Law.
Enhancement of the related design system
Applications for related designs can be filed up to 10 years from the filing date of the principle design. In addition, designs which are not similar to the principle design, but are similar to related designs can be registered.
Extension of term of design rights
Currently, the term of a design right is 20 years from the registration date. This term will be changed to 25 years from the filing date (the filing date of a principle design application in the case of a related design application).
Simplified filing/registration process of design applications
Multiple designs can be applied for in one application. The requirements for article names will be more relaxed and more flexible.
Expansion of provisions for indirect infringement
Under the current Design Law, the following act will indirectly infringe a design right: "Producing, assigning, leasing, or importing, or offering for assigning or leasing a product as business which is used only for manufacturing the article of a registered design or a similar design". However, if such a product has other uses and can be used for manufacturing other articles, it will not constitute indirect infringement. In order to strengthen design protection after the revision, producing, assigning, leasing, importing, or offering for assigning or leasing such a product as a business will indirectly infringe the design right if certain requirements are met.
Trademarks
Implementation of fast-track trademark examinations
In a bid to ensure prompt and accurate examinations, the JPO implemented a pilot programme for fast-tracking trademark examinations in October 2018, which is separate from the current accelerated examination process. Due to a surge in the number of trademark applications submitted to the Japanese Patent Office (JPO) for examination, the examination stage presently lasts around eight months, whereas it previously took only four to five months. If an application fulfills certain requirements described below, it will automatically be subject to fast-track examination. The examination will be two months shorter than regular examination.
Requirements for fast-track examinations
The requirements for fast-track examinations are:
(1) trademark applications designating only goods or services which are listed in the "Examination Guidelines for Similar Goods and Services", "Trademark Enforcement Regulations" or "International Classification of Goods and Services (Nice Classification)" at the time of filing the application;
(2) trademark applications for which no amendments to the goods or services have been filed prior to the start of the examination.
Custom seizure
The number of counterfeit products seized by Japanese customs has increased. In 2018, the number of cases was 26,500, and the number has exceeded 25,000 for seven consecutive years. In addition, the number of products seized reached 929,675 in 2018, marking the highest level for the fifth year in a row. In particular, there has been a surge in the number of products seized in relation to design rights. The above graph shows the recent trends in the number of products seized by customs.
The advent of new technical innovations, such as AI, has changed not only industrial structures but also the business climate surrounding intellectual property. The JPO's latest statistics show that the total number of patent applications filed with the JPO has slightly declined, whereas the number of applications filed by overseas applicants has slightly increased. This figure shows that Japan still holds a key position in the global market. The top echelons of companies would be wise to strategically protect and utilise their own IP rights as part of their corporate assets. Developing an IP strategy which is aligned with corporate strategy is key for companies to succeed in business while taking advantage of the above legislative changes and revisions to IP law.
Fig.1: Number of import seizures
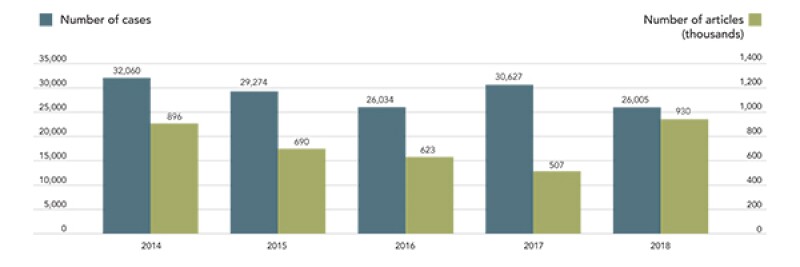
(Excerpted from the 2018 seizure statistics of IPR border enforcement by Japanese customs)
はじめに” to 日本における最新の知財動向
近年、人工知能(AI)を搭載した診断ロボット、オーダーメード医療等、技術のボーダレス化が進んでいる。また、ビジネスにおけるデザインの重要性が高まっている。さらに、激しい国際競争を勝ち抜くため、企業は経営戦略の一部として知的財産権の権利行使をより重視するようになった。このような状況下で、特許庁は、技術革新の波に対応し、出願人や権利者に有利な環境を構築すべく、知的財産権関連法を改正した。この記事では、主に2018年から2020年に施行・施行予定の知財法改正や最新の動向をまとめた。
特許
AI
AI関連の発明は、電気系分野のみならず最先端医療、機械など多岐にわたる技術分野で創出されている。2019年1月の審査ハンドブックの改訂では、AI関連発明に関する記載要件、及び進歩性に関して、事例を交えた記載内容が盛り込まれ、出願人による審査判断の予見性が高くなった。以下に留意点をまとめる。
記載要件
実施可能要件を満たすか否かは、教師データ内の複数のパラメータに相関関係があるかどうかで判断される。必ずしも発明の詳細な説明に具体的な相関関係が開示されている必要はないが、出願時の技術常識を考慮して相関関係が推認されない場合、実施可能要件を満たさない場合がある。また、AIによりある機能を持つと推定された物の発明は、生成された物の機能評価を実施例に記載するか、または学習済みモデルの予測精度の検証結果が記載されていないと、実施可能要件を満たさない。
進歩性
人間が行う業務の人工知能を用いた単純なシステム化や、推定手法の単純な変更の場合、進歩性は否定される。一方、機械学習に用いる教師データの追加に、顕著な効果が認められる場合や、機械学習に用いる教師データに対する前処理に進歩性が肯定されるような特徴があった場合、進歩性は認められる。
抗体医薬
抗体医薬は、現在、癌や希少疾患の次世代の治療薬として大きな注目を浴びている。抗体医薬を特許出願する際には、サポート要件を満たしているかどうか、つまり、実施例・比較例を記載する際にどの程度まで開示するか、という点に留意すべきである。医薬品発明において、低分子化合物やタンパク質の構造が複雑なため、サポート要件を満たすためには、できるだけ多くの実施例を記載することが必要である。しかし、一見、多くの実施例を開示したほうが請求の範囲が拡張できると思いがちだが、競合他社に対する情報の秘匿化、薬効に関係のない低分子化合物やタンパク質の大幅なデータ開示がかえって請求の範囲を狭める可能性があることも留意いただきたい。
例えば、2018年ノーベル医学生理学賞を受賞した癌免疫治療薬「オプジーボ」の特許を上記「実施例」に鑑みて、分析してみる。オプジーボ(ニボルマブ)は、主に非小細胞肺癌・腎細胞癌の治療薬として使用されている。ヒトPD-1(Programmed cell death-1)に対するモノクローナル抗体である。関連特許として、①特許第4409430号、②特許第5159730号、③特許第6035372号があるが、②および③は①を親出願とする分割出願である。①~③の請求項1の権利範囲はとても広く、PD-1抗体を有効成分として含むメラノーマ治療剤であれば、抗PD-1抗体を構成する重鎖及び軽鎖のアミノ酸配列に関係なく、全て請求項1の権利範囲に入る。
①~③は、抗PD-1抗体を抗がん剤として用いることに特許性を認められたものと考えられる。①~③のクレームをサポートする実施例では、1種類のみのモノクローナル抗体を用いたゼノグラフトモデルで転移抑制効果があることを確認しているものの、PD-1のノックアウトマウスを用いたがん細胞の移植実験で、がん細胞の増殖が抑制されるデータも出している。
しかしながら、他の抗体医薬関連の特許で、問題になっているように、どれだけ多くの実施例、本件では、何種類のモノクロナール抗体の薬効データを開示すべきかは議論の余地があるところと思われる。
訴訟
損害賠償額の算定基準変更
現行の特許法では、特許権者の生産能力等を超える場合は賠償が認められていない。しかし、改正後は、特許権者の生産能力を超える場合は、ライセンス相当額とみなされ、損害賠償を請求できる。加えて、ライセンス相当額を算定する際、特許権侵害があったことを前提として交渉した場合に決まるであろう額を考慮することができると明文化された。
意匠
2020年に改正意匠法が施行される予定である。デジタル技術を活用したデザインの保護や、ブランド構築に意匠権を活用しやすくすると共に、ユーザーフレンドリーな出願制度を実現することを目的とするものである。主なポイントを以下に概説する。
保護対象の拡充(画像、空間デザイン)
現在の意匠法では、画像は、物品の一部として保護され、それ単独では保護対象から除外されている。しかし、クラウド上に保存され、ネットワークを通じて提供される画像等を保護するニーズに対応するため、画像それ自体が新たに保護対象に加わることとなる。
更に、建築物の外観・内装のデザインも保護対象となる。
関連意匠制度の見直し
連鎖的な関連意匠の登録を認める等、関連意匠のより手厚い保護が可能となる。
意匠権存続期間の変更
意匠権の存続期間が25年に延長されるとともに、存続期間の終期の起算日が「意匠登録出願日(関連意匠の意匠権の存続期間は本意匠の意匠登録出願の日)」に変更となる。
意匠登録出願の簡素化(複数意匠一括制度、物品区分の見直し)
複数の意匠を一の願書で一括して出願することができる制度に変更となる。また、「意匠に係る物品」の欄の記載の要件が緩和される方向となる。
間接侵害規程の拡充
近年、意匠権を侵害する製品の完成品の構成部品を分割して輸入することにより、意匠権侵害を回避する等、模倣品の輸入手口が巧妙になっている。そこで、意匠法においても「多機能品型間接侵害」が導入される。
商標
ファストトラックの導入
2018年10月に、早期審査とは別に、試行的にファストトラック審査が導入された。商標の日本出願件数が急激に増加しており、審査期間を短縮化するためのものである。以下の要件を満たす案件は、通常より2か月程度早く最初の審査結果の通知が行われる。なお、申請、手数料は不要であり、要件を満たせば自動的にファストトラック審査の対象になる。
出願時に「類似商品・役務審査基準」、「商標法施行規則」又は「商品・サービス国際分類表(ニース分類)」に掲載の商品・役務のみを指定している商標登録出願
審査着手時までに指定商品役務の補正を行っていない商標登録出願
税関
近年、税関で押収される物品が増えている。2018年は26,005件で、7年連続で25,000件を超えている。輸入を差し止められた商品の数は、929,675点で、過去5年で最高水準となった。特に意匠権に関連した輸入差し止め実績は増加している。上記に税関差し止めの統計を示す。
まとめ
近年、知的財産を巡る動向はめまぐるしく変化している。日本特許庁への出願件数全体はここ数年は微減ではあるが、外国企業からの出願件数は微増しており、マーケットとしての日本市場が健在なことが伺える。上述した新たな施策・法改正を効果的に利用しつつ、自社の知的財産権を経営資産として戦略的に保護・利用することが重要である。
Masato Iida 飯田 雅人 |
||

|
|
Dr Iida specialises in biochemistry, molecular biology, and pharmaceuticals. He has extensive experience and expertise in prosecution, trials for invalidation, and suits against trial decisions in those fields. He is also qualified to represent parties in court in IP litigation. Dr Iida has experience in constructing target screening systems and evaluation systems for anti-cancer agents (both cell-based and cell-free systems) at a pharmaceutical company. He also has detailed knowledge of genome editing and antibody drugs, and frequently gives lectures and writes in medical magazines on these issues. 副所長 弁理士 農学博士 特定侵害訴訟代理弁理士 専門分野:化学・バイオ・製薬・訴訟・審判 学歴:東京大学大学院 農学生命科学研究科 応用生命工学専攻 職務経歴:製薬会社 主に医薬、バイオ系の出願、無効審判、審決取消訴訟を担当。前職では、抗癌剤のターゲット探索、スクリーニング系、評価系の構築(細胞系、無細胞系)の研究に従事。特にゲノム編集に関して造詣が深く、ゲノム編集のパテントマップと出願戦略における注意点等の講演実績がある。バイオと知財の豊富な知識・経験から、医学専門書への寄稿も行っている。 |
Tomohiro Gyoda 行田 朋弘 |
||

|
|
Tomohiro Gyoda handles prosecution cases, searches, oppositions and trials of designs or trademarks as well as enforcement cases relating to designs, trademarks and Unfair Competition Prevention Act. He has experience in cases in the field of information technology, such as protection of graphical user interfaces and trademark matters related to Classes 9 and 42 as he is a qualified advanced IT engineer. He also specialises in anti-counterfeiting, especially customs seizure. 弁理士 専門分野:意匠、商標、模倣品対策、不正競争防止法、ライセンス 学歴:東京外国語大学 外国語学部 欧米第一課程 ドイツ語専攻 職務経歴:知財専門翻訳会社、法律特許事務所 意匠・商標分野の出願、調査、異議、及び審判、並びに意匠、商標、又は不正競争防止法に関する権利行使関連業務を担当する。応用情報技術者の資格を有し、特に、画面デザイン等の情報技術分野における経験が豊富である。模倣品対策業務、特に税関差止も専門としている。 |










