Genentech, the plaintiff, owns a patent for an invention titled "vascular endothelial cell growth factor antagonists". Genentech filed an application for the registration of patent term extension in relation to the patent asserting that Genentech obtained an approval of partial changes in manufacturing approval (the disposition), which added a new dosage and administration for its medicine Avastin, whose general name is bevacizumab (the medicine). Regarding the medicine, there was a prior disposition that differs only in dosage and administration.
The JPO rejected the application and dismissed an appeal based on the Examination Guidelines because the prior disposition already existed for a medicine which had the same general name, effectiveness and efficacy as the medicine. Genentech appealed to the IP High Court seeking to revoke the decision of the JPO.
The IP High Court revoked the JPO decision and JPO appealed.
Judgment of November 17 2015, Supreme Court
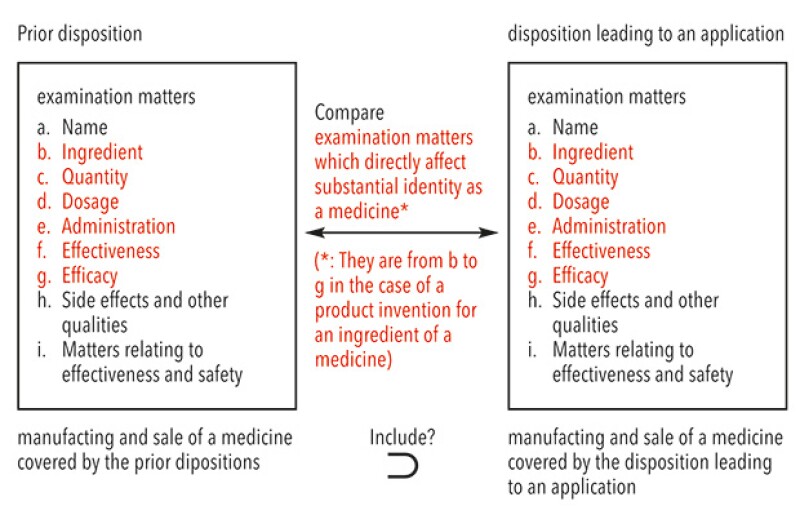
The Supreme Court clearly denied the Examination Guidelines, provided new criteria regarding the Patent Act Article 67-3(1)(i) and provided a new holding that applies to cases where there is a disposition leading to an application and a prior disposition. In such cases, if the manufacturing and sale of a medicine covered by the prior disposition includes that of a medicine covered by the disposition leading to an application, as a result of comparing both dispositions regarding examination matters which directly affect substantial identity as a medicine in light of a type or subject of a patented invention pertaining to the application of patent term extension, it is reasonable to deny that it was necessary to obtain the disposition leading to an application for the practising of the patented invention pertaining to the application for patent term extension.
Furthermore, the Supreme Court held that the patented invention was a product invention for an ingredient of a medicine and that examination matters which directly affect substantial identity as a medicine regarding a product invention for an ingredient of a medicine were their ingredient, quantity, dosage, administration, effectiveness and efficacy. The Supreme Court concluded that the manufacturing and sale of the medicine covered by the prior disposition did not include that of the medicine covered by the disposition leading to an application because the dosage and administration of the medicine differed from that of the prior medicine and although the prior disposition did not allow to manufacture and sell the medicine for combination treatment of Xelox treatment and bevacizumab treatment, the disposition made it possible for the first time. The Supreme Court upheld the IP High Court judgment.
Compared with Pacif Supreme Court and Avastin IP High Court judgments
The Supreme Court in the Pacif case only mentioned the case when the prior medicine is not included in the technical scope of the patented invention of the present patent. In contrast, this Supreme Court judgment made a new decision regarding the case when the prior medicine is included in the technical scope of the patented invention.
Although the IP High Court, the original instance, provided a concrete criterion as "a medicine that is identified by ingredient, quantity, dosage, administration, effectiveness and efficacy", this Supreme Court judgment provided more abstract criterion as "examination matters which directly affect substantial identity as a medicine". However, the Supreme Court judgment held that the examination matters to be considered in the case of a product invention for an ingredient of a medicine are ingredient, quantity, dosage, administration, effectiveness and efficacy. Therefore, the criterion which was finally adopted in the case is the same as the IP High Court. The abstract criterion of the Supreme Court can be interpreted to imply that the court can consider examination matters other than ingredient, quantity, dosage, administration, effectiveness and efficacy in the case of an invention other than a product invention for an ingredient of a medicine.
The original IP High Court judgment mentioned the scope of the effect of the extended patent right. However the Supreme Court did not mention it. Furthermore, although the original IP High Court judgment mentioned that the effect of extension can be redundant, the Supreme Court was silent on it.
Practical tips
This judgment makes it possible for the first time to be granted a patent term extension where a manufacturing and sales approval is issued for a medicine which has already been approved and the later approval differed in quantity, dosage or administration, even if ingredient, effectiveness and efficacy are the same as the prior disposition, if the different point is not "included" in the prior examination matters. (According to the current Examination Guidelines, it is usual that the patent term extension will be granted if the later approval differed in ingredient, effectiveness or efficacy.) This means that the patent term extension can be granted every time the dosage or administration is newly added to the medicine (the period of the extension does not exceed five years no matter how many times the patent is extended), however this Supreme Court judgment does not mention the scope of the effect of the extension. Therefore, it is unclear to what extent and how long the patentee can exercise the patent right when the extension is repeated. Further developments are expected.
The JPO's Examination Guidelines were revised in response to the Supreme Court judgment of the Pacif case. This Supreme Court judgment clearly denied the revised Examination Guidelines as well as the original IP High Court judgment. It has been announced that in response to this Supreme Court judgment the JPO will re-revise the Examination Guidelines and that these will be published in spring 2016.

|

|
Takanori Abe |
Michiko Kinoshita |
ABE & PartnersMatsushita IMP Building1-3-7, Shiromi, Chuo-ku, Osaka, 540-0001, JapanTel: +81 6 6949 1496Fax: +81 6 6949 1487abe@abe-law.comwww.abe-law.com










