Three years ago, Korea adopted a new patent term adjustment (PTA) system. As many readers know, PTA is a system that allows extension of a patent term to the patent owner to compensate for an unreasonable delay during the prosecution phase.
The duration of a patent term is of interest to every patent owner. However, in some industries (such as pharmaceuticals), it is not just a matter of interest, because it may be the most important aspect of the patent. Maximising the protection period is a key factor in patent portfolio management for drug developers. For that reason, it is worth knowing how the Korean system is structured, how it is different from other jurisdictions and how to get an advantage from this complicated system.
So why is it important to think about the PTA, now, in 2016? The answer is, because the present time is the earliest period when a patent owner can enjoy the benefit from the new PTA system. Even though the PTA was adopted in 2012, patents can actually enjoy this benefit starting from March 15 2016 because a request for PTA can be made for patents which were issued later than: (a) four years after the filing date or (b) three years after the date of requesting examination, whichever is later.
The extendable period to a normal patent term, which is 20 years from the filing date, is calculated by the following simple equation (see also the figure):
Extendable Time = [from reference date to Issue date] – [delay attributable to the applicant].
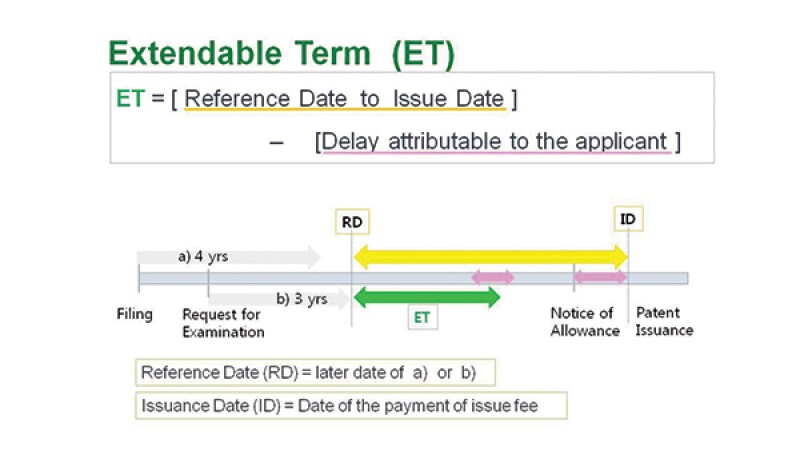
The reference date is a later date of (a) or (b) above.
Regarding the delay, the Enforcement Decree of the Korean Patent Act lists more than 40 such situations where the period of delay may be deducted from the extendable time period.
One important point of emphasis about the Korean PTA system is that the patentee must file a separate request to KIPO because it is not automatically awarded to the patentee. KIPO does not calculate the extendable term or notify the patentee of its eligibility. Further, the request must be made within three months from the date of paying the issue fee. The procedure thereafter is similar to the procedure of a regular patent application. After examining the PTA application, KIPO will issue an office action if it determines that the application does not meet the statutory requirements.
Another thing to keep in mind is the period it takes to respond to the office action. It is important to note that this responding time period may be deducted from the extendable time period if an amendment was filed. This is the case even if the applicant timely filed a response to the office action. However, if the patent was granted, without an amendment, based only on the written statement or written argument, this responding time period will not be deducted from the extendable time period. This feature is somewhat unique in the Korean system.
In addition to the PTA, Korea has another way of extending the patent protection – patent term extension (PTE). PTE allows drug developers to extend the duration of the patent term by up to five years, whereas PTA applies to patents in all technical fields. Therefore, with the PTA in conjunction with the PTE, patent owners can now enjoy a more comprehensive protection of their patent rights in Korea than ever before.
Min Son
Partner, Hanol IP & Law
HANOL Intellectual Property & Law
6th Floor, 163, Yang Jae Cheon-Ro, Gang Nam-Gu
Seoul 06302, Republic of Korea
Tel: +82 2 942 1100
Fax: +82 2 942 2600












