While the proposal for an EU reform on standard-essential patents (SEPs) has been shelved, the reform of the supplementary protection certificate (SPC) system is ongoing. It aims at the creation of a more streamlined and unified SPC framework aligned with the unitary patent system.
In April 2023, the European Commission submitted four regulatory proposals for reforming the SPC regime regarding plant protection products and medicinal products. Regarding medicinal products, the Commission issued the following two proposals:
A proposal for a regulation on the SPC for medicinal products (COM(2023) 231) – this proposal is a recast of Regulation (EC) No. 469/2009. Chapters I and II mainly contain the articles of the current regulation, including amendments regarding substantive aspects such as third-party marketing authorisations (MAs) and several SPCs for one product. Chapter III contains rules defining a centralised grant procedure and the according granting bodies.
A proposal for a regulation on the unitary SPC for medicinal products (COM(2023) 222) – this proposal contains all the regulations defining the procedure regarding unitary SPCs.
In February 2024, the European Parliament approved the two proposals, with some amendments. Trilogue negotiations – i.e., informal inter-institutional meetings between the Council of the European Union, the European Parliament, and the European Commission – are ongoing regarding the design of the system of legal remedies and the designation of the authority responsible for granting unitary SPCs. If successful, these negotiations will be followed by a second reading and final adoption before the new SPC regulations can enter into force.
The existing SPC grant procedure
Currently, the national patent offices (NPOs) are responsible for granting SPC rights based on several types of basic patents and MAs under Regulation (EC) No. 469/2009. Presently, a basic patent may be:
A national patent;
A unitary patent; or
A national patent validated from a European patent (EP).
The MA can be granted centrally by the European Medicines Agency (EMA) or by the national health authorities. Opposition proceedings do not exist for SPCs under Regulation (EC) No. 469/2009. Although third-party observations (TPOs) may be considered by most of the NPOs during the SPC grant proceedings, there are no respective rules in the current SPC regulation.
The new SPC grant procedure
Under the new regime as defined by the two proposals, SPC applications relying on an EP (a unitary patent or a national patent validated from an EP) and a centralised MA granted by the EMA will be examined by the EUIPO in Alicante. Applications via an NPO are excluded for such applications based on an EP and a centralised MA.
The application must be published within five working days after compliance with the formal requirements of the EUIPO.
The EUIPO will then issue a positive or negative examination opinion regarding the grant of the SPC. The EUIPO is required to issue an examination opinion within six months after publication of the SPC application. Where an urgent need for examination can be demonstrated, the SPC applicant may request an expedited examination. In such instances, the EUIPO must issue an examination opinion within four months.
The substantive examination process will be carried out by an examination panel consisting of one EUIPO official and two examiners appointed from different NPOs. At least one of the national examiners must have a minimum of five years’ experience in examining patents and SPCs.
TPOs must be submitted within three months of the publication of the application, or within six weeks in expedited procedures. These TPOs will be notified to the applicant.
Within two months after the publication of the examination opinion, a notice of opposition can be filed by third parties, which must include grounds laying out that the conditions for obtaining a certificate or conditions regarding entitlement to the certificate are not fulfilled, and supporting evidence. The opposition will be examined by an opposition panel within the EUIPO.
If a positive examination opinion is issued, after the expiry of the appeal or opposition period or after a final decision has been issued, national SPCs are then formally granted by the NPOs of the designated member states for which the SPCs have been requested and/or if a single unitary SPC was applied for, this unitary SPC is granted by the EUIPO.
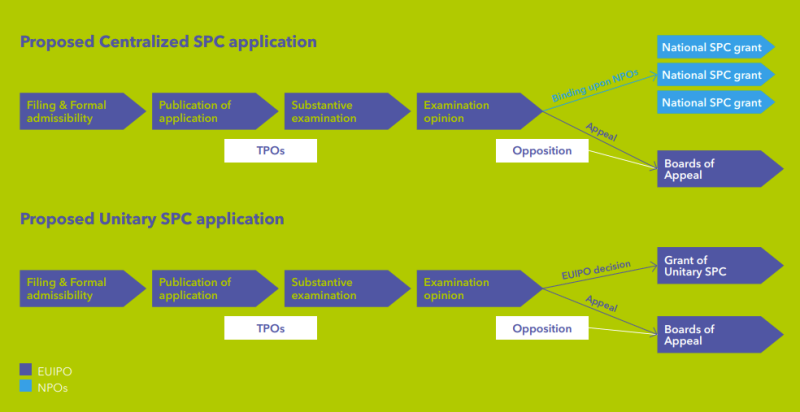
Types of SPC applications under the new system
Applicants will have different filing options under the new system:
A unitary SPC application will be available exclusively for products that have received a centralised MA from the EMA, are based on a unitary patent, and will be examined and granted by the EUIPO.
A single centralised SPC application, which can be based on an EP (a bundle patent or unitary patent) and a centralised MA from the EMA, will result in a bundle of national SPCs in the designated EU member states. The examination will be conducted by the EUIPO, and national SPCs will ultimately be granted by national offices.
The centralised SPC application may include a request for a unitary SPC application. This so-called combined application leads to a unitary SPC covering all states where the unitary patent is in effect, along with a bundle of national SPCs in the remaining designated EU member states. The examination is conducted by the EUIPO, and the unitary SPC will be granted by the EUIPO, while the national SPCs will the granted by NPOs.
In the uncommon case that the SPC is based on a national MA, the ‘old route’ would still be available via examination by the respective NPO. Such an SPC could be based on a national patent or an EP (including a unitary patent in force in the corresponding national country).
Changes to the substantive aspects of the SPC system
Although the European Commission initially claimed that the proposed legislation would not alter the substantive aspects of the existing SPC regime, some notable changes have been introduced. Article 3 (Article 3(3) of the centralised SPC procedure regulation proposal and Article 3(2) of the unitary SPC procedure regulation proposal) now states that multiple SPCs for the same product may be granted if filed by different patent holders, provided they are not “economically linked”.
A new definition of economically linked entities in respect of different holders of two or more basic patents protecting the same product has been introduced, stating that companies are considered economically linked if one controls, is controlled by, or is under common control with the other entity. This clarifies that independent companies that have agreed on a licensing agreement should not be regarded as economically linked and may each obtain an SPC for the same product based on their respective patents.
Additionally, Article 6(2) (Article 6(2) of the centralised SPC procedure regulation proposal and the unitary SPC procedure regulation proposal) has been introduced into the new regime, stipulating that an SPC can only be granted if the MA holder has given explicit consent when the authorisation is held by an entity differing from the patent proprietor. This consent has to be filed together with the SPC request. Thereby, so-called SPC squatting based on a third party’s MA without the consent of the third party is explicitly excluded in the future regime.
No further changes were made regarding the substantive aspects. Of note, the recitals of the proposed regulations newly incorporate relevant citations regarding Article 3 from the case law of the Court of Justice of the European Union.
Next steps
Trilogue negotiations are ongoing to align upon the key points. It remains to be seen whether these negotiations succeed or whether the SPC reform will suffer the same fate as the SEP regulation to be shelved since no agreement could be reached. If an informal agreement is achieved, the proposals must then be approved according to the rules of procedure of each of the institutions so that the new SPC regulations can enter into force.
For example, at present there are doubts regarding the suitability of the EUIPO as the examining authority (see, for example, European Patent Institute position papers from April 2024 and November 2024). At present, the EUIPO primarily deals with trademarks and designs and has no experience in examining patents. Some voices advocate for the EPO to take on this role, as it has an established patent register, an efficient language regime, and examiners experienced in patent assessment. Additional concerns have been raised regarding the fragmented system for invalidation proceedings that may result from the current proposal, which will be analysed in greater detail in a subsequent Maiwald article.












