A new patent term extension (PTE) system will be implemented in South Korea starting July 22 2025. The extension will be capped at 14 years from the date of marketing approval (MA), and the number of patents that can be extended will be limited to one. This new regulation applies to PTE applications filed on or after July 22 2025.
Background
Unlike other jurisdictions such as the US, Europe, and China, Korea has previously allowed PTEs without a 14- or 15-year time limit from the date of MA and for multiple patents involved in a single MA. However, concerns have been raised that this broad protection delays generic entry and limits public access to affordable medicines. The latest revisions to the Patent Act are aimed at addressing this issue.
Fourteen-year cap from the date of MA
In Korea, the length of an extension corresponds to the period during which a patented invention covering a drug or agrochemical product could not have been practised after the patent issuance due to the MA for the product.
In practice, the length of the extension is calculated as the sum of:
The time taken for domestic clinical trials (i.e., clinical trials conducted with the approval of the Korean regulatory authorities); and
The time taken for regulatory approval review, except for periods occurring before the date of patent issuance.
Any delay attributable to the MA holder (such as a data supplementation period) is excluded from the extendible period. The maximum five-year extension is available.
Under the amended Patent Act, there is no change in the calculation practice mentioned above, but an additional limitation applies: the total extension cannot exceed 14 years from the date of MA (Article 89(1) of the Patent Act).
The following hypothetical example, where the total extension exceeds 14 years from the date of MA, explains that the extended expiry date will be shortened under the amended Patent Act.
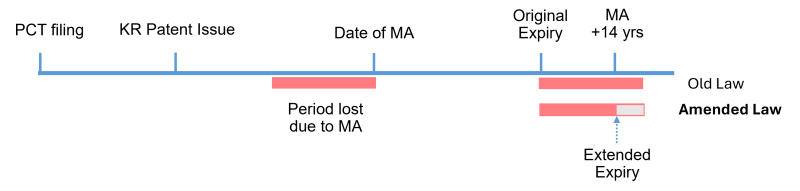
In cases where the extensions expire within 14 years from the date of MA, there is no reduction due to the 14-year cut-off.
Meanwhile, if a patent has an original expiry date that is more than 14 years from the date of MA, the patent is not eligible for a PTE.
Violating the new limitation will constitute grounds for rejection or invalidation. PTE applicants can resolve this issue by correcting the extension period voluntarily or when responding to the examiner’s office action.
Only one patent extension per approval
Korea now has the following limitations on PTE granting:
A PTE is granted only once per patent;
If there are multiple MAs for multiple active ingredients covered by a single patent, a PTE is granted only once for the patent, based on one selected from the multiple MAs; and
If there are multiple MAs for the same active ingredient, a PTE is granted only once for a single patent, based solely on the first MA.
Under the previous system, if multiple patents are involved in a single MA, there was no limitation on the number of patents eligible for a PTE, so all the patents could benefit from a PTE.
Now, under the amended system, a new limitation is added: only one patent is eligible for a PTE per approval (Article 90(7) of the Patent Act). Violating the new limitation will constitute grounds for rejection or invalidation. Thus, PTE applicants must ultimately elect one patent for the extension.
The decision to elect a patent for a PTE can be made before the PTE filing deadline, which is three months from the date of MA. Alternatively, this decision can be postponed after filing PTE applications for multiple patents until the issuance of the examiner’s office action for violating this limitation. When deciding this, the patent holders will need to make strategic considerations to maximise the PTE.
Strategic considerations
Under the new system, it is important to decide which patent to extend. Many factors need to be considered, such as:
Which patent can ensure the longest patent term within the 14-year cap;
Which patent is the strongest or most vulnerable against validity challenges;
Which patent is the easiest to enforce;
The subject matter (product claim versus method claim);
The scope of the patent; and
Whether the patents are primary or secondary patents.
For example, let us assume that there are four patents involved in a drug product X for which MA is expected to be obtained on July 26 2025. Their calculated PTE days and final expiry dates are shown in the table below.
| Claimed subject matter | Patent Cooperation Treaty filing date | Original expiry date | Calculated PTE (days) | Projected final expiry date | Elect? |
Date of issuance | ||||||
Patent 1 (P1) | Compound A
| 17/1/2017 | 17/1/2037 | 1,031 | 14/11/2039 > 14-year cap = 26/7/2039 | Yes |
11/10/2021 | ||||||
Patent 2 (P2) | Medicament for treating disease Y comprising compound A | 20/2/2018 | 20/2/2038 | 478 | 13/6/2039 < 14-year cap | No |
28/12/2023 | ||||||
Patent 3 (P3) | Sustained release formulation comprising compound A | 19/5/2019 | 19/5/2039 | 246 | 20/1/2040 > 14-year cap = 26/7/2039 | No |
16/8/2024 | ||||||
Patent 4 (P4) | Medicament for treating disease Y in a specific patient population with a specific dose, comprising compound A | 20/5/2020 | 20/5/2040 > 14-year cap | 0 | N/A | No |
10/1/2025 |
First, the 14-year cap is July 26 2039, and the maximum extension may therefore be until July 26 2039. Any patents with original expiry dates later than the 14-year cap should be excluded, since they are not eligible for a PTE (e.g., P4 in the above table).
In the above cases, while various factors should be considered, selecting the one claiming the product (e.g., P1) might be a safe approach, as product claims are generally easier to defend and enforce.
Meanwhile, under the new rule of one patent extension per one approval, it is still important to file divisional applications if a parent application covers multiple drug candidates so as to ensure that a PTE can be filed for each potential active ingredient. Furthermore, to maximise a PTE, using an accelerated examination may be a strategic approach because only the non-working period (of the domestic clinical trial and regulatory review) ‘after’ the date of patent issuance is counted for a PTE.












