Methods for treating humans or animals by surgery or therapy and diagnostic methods practised on the human or animal body are excluded from patentability under Article 53(c) of the European Patent Convention (EPC). However, according to Article 53(c) of the EPC, this provision does not apply to “products, in particular substances or compositions, for use in any of these methods”.
Article 54(4) and (5) of the EPC specify that a claim directed to any “substance or composition”, comprised in the state of the art, for use (first medical use) or for any specific use (second medical use) in a method referred to in Article 53(c), will be held novel, provided that the use is not comprised in the state of the art. In other words, if the claim refers to a “substance or composition” in the sense of Article 54(4) and (5) of the EPC, the claimed use in a method referred to in Article 53(c) is considered a limiting feature to the claim (‘purpose-limited product claim’).
Thus, the question of which products qualify as a substance or composition is an essential one.
The practice according to the Guidelines for Examination
The Guidelines for Examination in the European Patent Office (the Guidelines), in G.VI.7.1.1, stipulate that the above principles only apply to “substances and compositions” as also expressly referred to in Article 54(4) and (5) of the EPC (although Article 53(c) of the EPC uses a broader definition – namely, “products, in particular substances or compositions”) – and cannot be extended to other products.
The Guidelines, with reference to decision T 1758/15 of July 11 2017, state that a product only qualifies as a substance or composition if it is the active agent or ingredient in the specific medical use and if the therapeutic effect can be ascribed to its chemical (and not merely physical/structural) properties. Otherwise, the product qualifies as a medical device rather than a substance or composition.
A claim directed to a device for an intended medical use would be construed as being directed to a device that is merely suitable for that medical use. The medical use per se would not be considered a limiting feature of the claim, which would then lack novelty in the event that the device is already known.
The board’s view in T 1252/20
On February 6 2024, EPO Board 3.3.10 (the Board) issued a new decision, T 1252/20, on substances and compositions in the context of medical use claims.
The application in T 1252/20 was directed to self-assembling, amphiphilic peptides applied as an aqueous solution into a blood vessel. Those peptides form a hydrogel in situ upon contact with body fluid. This hydrogel then blocks the blood vessel, which induces necrosis of the tissue (T 1252/20, reason 2). Claim 1 was directed to compositions comprising solutions of those specific peptides for use in treating cancer, for example (T 1252/20, reason 3). The peptide solutions according to claim 1 were already known in the art (T 1252/20, reason 4).
The examining division, with reference to the Guidelines (G.VI.7.1.1) and decision T 1758/15 cited therein, held that the peptide solutions defined in the claim did not qualify as a substance or composition in the sense of Article 54(5) of the EPC but rather constitute a device, as the mode of action was purely physical and based on the macroscopic 3D structure (the hydrogel formed in the body obstructed the blood vessel). Thus, the use defined in the claim was not a limiting feature and the claim consequently lacked novelty (T 1252/20, reasons 5 and 6.2).
The Board, however, was convinced that the peptide solutions claimed do qualify as a substance or composition and that the claimed subject matter was thus novel over the prior art. It based its finding not on reasoning aligned with T 1758/15 and the chemical mode of action requirement but considered the justification to be based on more fundamental reasons (T 1252/20, reasons 5 and 6.5).
In its analysis, the Board focused on delimiting the claimed product from a device, which clearly is not covered by substance or composition (T 1252/20, reasons 6.1 and 7.1), but otherwise took a very liberal approach in view of the fact that (i) not only therapy but also surgery and diagnostic methods are covered by Article 54(4) and (5) of the EPC and require criteria other than the chemical mode of action (T 1252/20, reason 8.2), and (ii) the lack of a legal basis for the chemical mode of action requirement (T 1252/20, reason 9).
The Board emphasised that while devices such as a pacemaker or a surgical scalpel do not qualify as a substance or composition, there is no apparent reason to disqualify a solution of a peptide – i.e., a shapeless liquid defined without any device-like features – from the scope of Article 54(5) of the EPC, even if, once used as defined in the claim, the peptide solution will transform into something that may act as if it were a device (T 1252/20, reasons 7.2 and 11).
The Board held that the question of whether a product is a substance or composition in the sense of articles 53(c) and 54(4) or (5) of the EPC should be decided on the basis of the claimed material or object as such. If this analysis leads to the conclusion that a substance or composition is indeed present, the requirement of Article 54(4) or (5) of the EPC is fulfilled. No additional restrictions relating to its mode of action are derivable from the EPC (T 1252/20, reason 12).
The Board discusses several decisions in this context, an overview of which is given below.
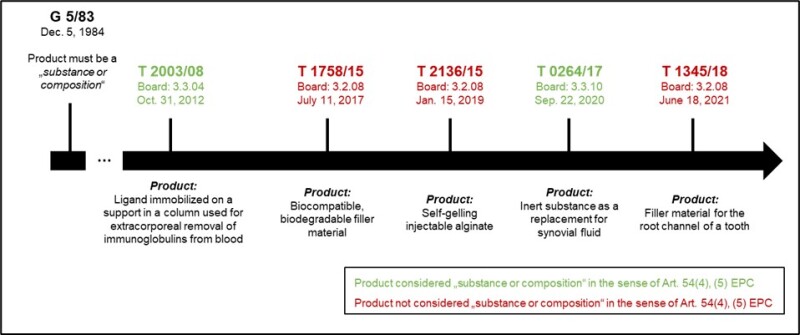
Analysis of the decision
In T 1252/20, the Board found that there is no legal basis for the chemical mode of action as a criterion for qualifying a product as a substance or composition. This finding is in contrast to the requirements for a product to be qualified as a substance or composition stipulated in the Guidelines (G.VI.7.1.1), both in the version as of March 1 2023 and the new version of March 1 2024, as well as other case law.
Following the Board’s view in T 1252/20 would allow applicants to draft medical use claims to a broader range of materials or objects already known in the art.













