The fourth amendment to China’s Patent Law (Article 42) introduced a patent term extension (PTE) regime in China. The newly revised Implementing Regulations of the Patent Law and the Guidelines for Patent Examination, which will enter into force on January 20 2024, flesh out the much-needed details of the regime.
Article 42 of the Patent Law prescribes two types of PTE:
An extension to compensate for an unreasonable delay in the prosecution process of an invention, which is the Chinese counterpart of the US patent term adjustment (PTA) mechanism (see part I); and
An extension to compensate for the time required for obtaining administrative approval to market a new drug, which is the Chinese counterpart of the US PTE mechanism (see part II).
Part I: PTA 101 in China
Invention patents that have experienced an unreasonable delay in the prosecution process are eligible for a PTA, unless the applicant files on the same day an invention patent and a utility model for the same invention, and the invention patent is later granted (Article 9 of the Patent Law).
In principle, the request for a PTA shall be made by the patentee to the CNIPA within three months from the date of grant. In the transitional period between the entry into force of the fourth amendment of the Patent Law (June 1 2021) and that of the newly revised regulations (January 20 2024), an invention patent granted after June 1 2021 is eligible for a PTA (where examination commences after January 20 2024), provided that the PTA application is submitted within three months from the date of grant. Where a patent that is eligible for a PTA has expired before January 20 2024, a PTA can still be granted, with the extended term commencing on the original expiry date.
The extended term of a PTA shall be calculated as follows:
Extended term = unreasonable delay in the prosecution process – reasonable delay – unreasonable delay caused by the applicant
Unreasonable delay in the prosecution process
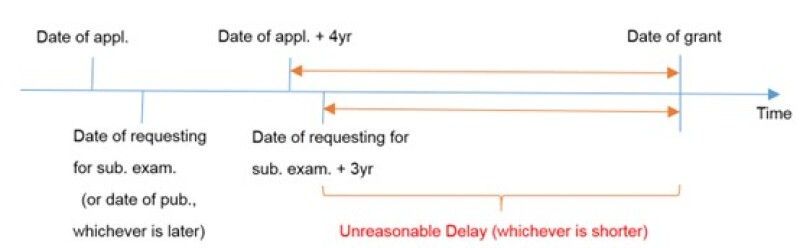
An unreasonable delay in the prosecution process refers to an undue delay in the date of grant. It is defined as the interval between “four years after the date of application”, “three years after the date of requesting substantial examination”, “three years after the date of publication”, whichever is later, and the date of grant.
For Patent Cooperation Treaty (PCT) applications and divisional applications, the date of entry into the national phase and the date of filing divisional application shall be deemed as the date of application respectively.
Reasonable delay
A reasonable delay shall be deducted from the extended term. The regulations set forth the below circumstances as qualifying as a reasonable delay:
Where the applicant has revised the application documents in filing a re-examination request or in response to the notification of re-examination in a re-examination proceeding;
Where the examination has been suspended due to disputes over the right to file a patent application;
Where the court has ruled to take preservation measures against the right to file a patent application; and
Other eligible circumstances.
Unreasonable delay caused by the applicant
An unreasonable delay caused by the applicant (as shown below) shall also be deducted from the extended term:
Where the applicant has failed to respond to an office action in due time;
Where the applicant has requested for deferral of examination;
Where the applicant has requested incorporation by reference;
Where the applicant has requested restoration of right; or
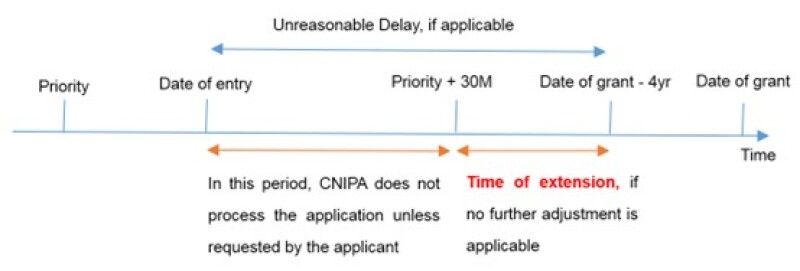
Where the applicant has not requested to process a PCT application prior to the expiry of 30 months from the priority date.
Article 23 of the PCT prescribes that a designated office shall not process or examine an international application prior to the expiry of 30 months from the priority date, in the absence of an explicit request by the applicant. Where the applicant does not make such a request, the interval between the date of entry into the national phase and the expiry of 30 months from the priority date shall be deducted from the extended term.
Examination and remedy
The examination of a PTA shall follow the principle of hearing; i.e., the applicant shall be given at least one opportunity to make observations and/or amend the documents.
The patentee and the third party that is involved in a patent infringement dispute may apply to the CNIPA for administrative reconsideration against a decision to grant/not to grant a PTA.
Part II: PTE 101 in China
Invention patents seeking protection over products, methods of preparation, or medical uses (Swiss-type) that are associated with approved innovative drugs or certain modified new drugs are eligible for a PTE.
In accordance with the drug registration classification system of China’s National Medical Products Administration, patents associated with drugs in the following categories are eligible for a PTE.
For traditional Chinese medicine drugs or natural drugs:
Innovative drugs; and
Modified new drugs treating new indications.
For chemical drugs:
Innovative drugs that have not yet been marketed in China or overseas;
Modified new drugs that are the esterification or salification of known active ingredients; and
Modified new drugs treating new indications.
For biologicals:
Innovative vaccines and innovative biologicals for a therapeutic purpose;
Modified vaccines utilising a new virus seed; and
Modified biologicals treating new indications.
A novel drug that has been marketed overseas before filing an application for marketing approval in China will not be deemed as an innovative drug, and the pertinent patents are therefore not eligible for a PTE in China.
On top of that, a patent that is eligible for a PTE shall meet the following conditions:
The date of grant of the patent pre-dates the date of obtaining the marketing approval of the drug implementing the patent;
The patent is valid;
The patent term has not been extended by a PTE;
A claim of the patent incorporates the technical solution of a drug that has obtained marketing approval;
Where the drug implements multiple patents, only one patent may be granted a PTE; and
Where a patent is implemented by multiple drugs, application for a PTE may be filed for only one of the drugs.
Application time limit
A request for a PTE shall be made by the patentee to the CNIPA within three months from the date of obtaining marketing approval. If the patentee is not simultaneously the holder of the marketing approval, written consent of the latter shall be obtained.
During the PTE of a patent, the protection scope of the patent shall be limited to the technical solutions of the approved new drug and the approved indications.
In the transitional period, a patent for which a PTE application is filed after June 1 2021 is eligible for a PTE, provided that the PTE application is filed within three months from the date of obtaining the marketing approval of the drug implementing the patent.
Calculation of the extended term
In practice, the patentee may request both a PTA and a PTE, should their invention experience an unreasonable delay in the prosecution process, and the patentee went through a lengthy procedure to obtain administrative approval to market new drugs implementing the invention. In such case, the length of the PTE shall be determined on the basis of the duration of the PTA, unless the patentee explicitly waives the PTA.
The term of a PTE, which is subject to the provisions of Article 42(3) of the Patent Law, is determined by deducting five years from the interval between the filing date of the patent and the date of obtaining marketing approval in China for the new drug. Specifically, the extended term is capped at five years, with the total validity period after obtaining the marketing approval capped at 14 years.
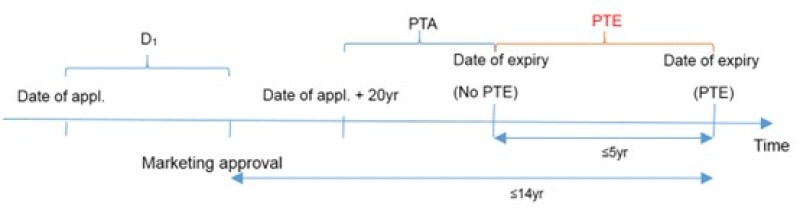
It can be observed from the above time axis that:
PTE = the time between the date of application and the date of obtaining marketing approval (D1) – 5 years;
PTE ≤ 5 years; and
The interval between the date of obtaining marketing approval and the original date of expiry (20 – D1) + PTA + PTE ≤ 14 years
It can be deduced that:
If D1 – PTA ≤ 6 years, a PTE cannot be obtained;
If 6 years < D1 – PTA ≤ 11 years, the term of the PTE equals D1 – PTA – 6 years; and
If D1 – PTA > 11 years, the term of the PTE equals 5 years.
Examination and remedy
The examination and remedy of a PTE is analogous to that of a PTA, except that a party that is eligible to apply for an administrative reconsideration could include a third party that has filed an application to obtain the marketing approval for a relevant drug.
Final comment on the PTA and PTE regime in China
It remains to be seen how the PTA and PTE regime will be implemented in China. Innovative and generic drug makers are advised to watch the CNIPA’s case law closely to see if the agency will provide any further guidance on this matter.












