On August 10 2023, the China National Intellectual Property Administration (CNIPA) issued two invalidation decisions, No. 561725 (Decision #1) and No. 562232 (Decision #2), in response to invalidity requests filed by the same petitioner against AbbVie’s two core patents related to its blockbuster drug upadacitinib.
Decision #1 declared the patentee’s compound patent ZL201080062920.6 invalid in its entirety. Decision #2 maintained the partial validity of the patentee’s pharmaceutical composition patent ZL201810902092.0, keeping alive the claims relating to a general formula covering upadacitinib, yet declaring the claims related to upadacitinib invalid.
Both rulings are based on the same facts and reasoning. The decisions, which have thrust AbbVie and the Chinese patent portfolio of upadacitinib into the limelight, are appealable before the Beijing Intellectual Property Court until November 10 2023.
Other than the aforesaid patents, AbbVie also owns a crystal patent, ZL201680070259.0, for upadacitinib in China. Assuming that AbbVie appeals, and the Chinese judiciary affirms the invalidation decisions in the ensuing administrative litigation, generic manufacturers seeking to market generic versions of upadacitinib would still need to clear the hurdle posed by what is left of the partially invalidated composition patent. Nonetheless, the CNIPA’s decisions deal a heavy blow to AbbVie’s upadacitinib patent portfolio.
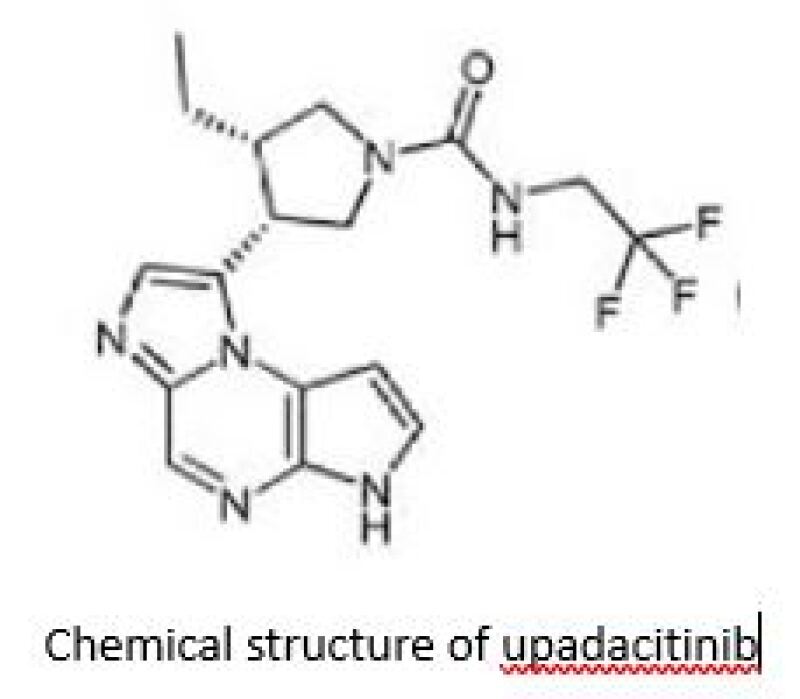
Marketed as RINVOQ, upadacitinib is an oral selective Janus kinase 1 (JAK1) inhibitor first approved by the US Food and Drug Administration in August 2019. In January 2023, upadacitinib was included in China’s National Reimbursement Drug List after AbbVie secured marketing approval from the National Medical Products Administration in February 2022. In China, upadacitinib has been approved to treat five indications, including moderate to severe rheumatoid arthritis, active psoriasis arthritis, moderate to severe active ulcerative colitis, and moderate to severe active Crohn's disease.
On October 12 2023, AbbVie announced that its phase 2b study investigating upadacitinib for the treatment of adults with non-segmental vitiligo had met the primary endpoint and it will be advancing the clinical trial programme of upadacitinib for vitiligo to phase 3.
Statistics indicate that AbbVie generated $2.52 billion from global sales of upadacitinib in 2022, and the momentum is expected to continue, with sales projected to exceed $7.5 billion in 2025.
Invalidation proceeding
An invalidity action was brought by Sichuan Gowell Pharmaceutical Co., Ltd. (Gowell) on December 30 2022. Gowell, which intends to market a generic version of upadacitinib in China, registered a clinical trial to test the bioequivalence of upadacitinib generics, which was publicised on the Drug Clinical Trial Registration and Information Disclosure Platform on August 31 2023.
This article analyses Decision #1, which invalidates AbbVie’s compound patent ZL201080062920.6, titled ‘novel tricyclic compounds’.
In the invalidation request, Gowell mainly argued that:
The claims of upadacitinib were not sufficiently disclosed in the description of the subject compound patent, as no experimental data for upadacitinib was documented and a person skilled in the art could not determine or expect the technical effect to be achieved; and
The claims of upadacitinib were devoid of inventiveness. Upadacitinib could not benefit from an earlier priority date of December 1 2009 due to the absence of description in the earlier priority document, thus the application date of the subject compound patent should be postponed to a priority date of July 14 2010. Consequently, Evidence 2, which is another prior Patent Cooperation Treaty application filed by AbbVie with a date of publication of December 17 2009, shall be the closest prior art for upadacitinib. Therefore, upadacitinib was not inventive relative to Evidence 2.

As counterevidence, AbbVie submitted the inventor's statement regarding experimentation, and the supplementary experimental data furnished in the opposition procedure of the EPO related to the European family patent of the subject compound patent. In the opposition procedure, the EPO affirmed the technical effect, which the supplementary experimental data intended to prove, and ascertained the inventiveness of upadacitinib.
Based on the counterevidence, AbbVie contended that upadacitinib had a better inhibitory effect on JAK1, and could achieve the selective inhibition of JAK1/3, compared to some compounds described in Evidence 2 as the closest prior art; thus, upadacitinib was sufficiently disclosed in the description of the subject compound patent and was non-obvious over Evidence 2.
The CNIPA found the arguments untenable and declared the compound patent invalid on the ground of lack of inventiveness.
CNIPA reasoning
The CNIPA delved into the inventiveness assessment of upadacitinib using a layered approach.
With regard to the priority assessment, the CNIPA analysed the earlier priority document dated December 1 2009, which introduced a Markush structure formula.
Given that a Markush structure formula should be deemed as a collection of Markush elements, rather than a collection of many compounds, upadacitinib, which falls within the scope of the Markush structure formula introduced, was not explicitly described in the priority document. Therefore, upadacitinib could not claim the earlier priority. The application date of the subject compound patent should be postponed to the priority date of July 14 2010, and Evidence 2 is the prior art to upadacitinib.
The CNIPA further found that the subject patent and Evidence 2 are highly relevant, as they share identical expression on background art, invention purpose, and technical effects such as correlation between JAK enzymes and indications. On top of that, upadacitinib falls within the scope of chemical formula Ic as described in the subject patent and Evidence 2, and the two chemical formulas are mostly overlapping. Therefore, whether upadacitinib is non-obvious over Evidence 2 hinges on the comparison of their technical effects.
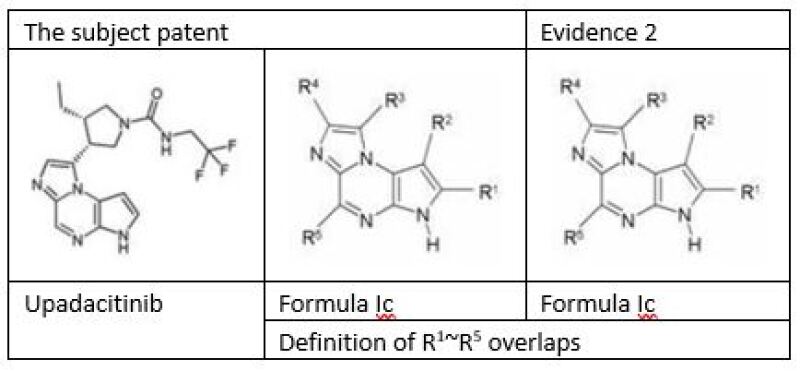
With respect to technical effects, the CNIPA observed that Evidence 2 did not furnish any experimental data, while the subject patent documented the activity data of a total of 185 specific compounds (exclusive of upadacitinib) on the JAK3 enzyme. The subject patent merely documented the information related to the preparation of upadacitinib, without any activity experimental results. It would be evident to a person skilled in the art that the subject patent focuses on the inhibitory activity for the JAK3 enzyme. However, a person skilled in the art would anticipate at best that upadacitinib has a JAK3 inhibitory effect similar to that of the compounds tested in the description of the subject patent, and that the compounds covered by the chemical formula Ic (inclusive of upadacitinib) of Evidence 2 should also have roughly equivalent activity.
In assessing the admissibility of supplementary experimental evidence, the CNIPA explicitly stated that “the matter surrounding the admissibility of supplementary experimental evidence should be approached with prudence by applying the principle that 'the facts and/or technical effects to be proven by supplementary experimental evidence should be obtained by a person skilled in the art from the disclosed content of the patent application.'”
AbbVie adduced five sets of supplementary experimental data to prove the unexpected technical effects of upadacitinib. The CNIPA held that the inventor's statement on experiments conducted on October 19 2010, prior to the application date of the subject patent, recorded the inhibitory effects of seven compounds on JAK3, including upadacitinib. As control compounds in the statement do not fall within the scope of chemical formula Ic of Evidence 2, the experimental results cannot represent the difference in terms of inhibitory effects on JAK3 between upadacitinib and the overall technical effect of the closest prior art.
Another inventor's statement on experiments conducted during 2009–11 – in combination with the supplementary experimental evidence submitted in the European patent opposition procedure, based on which the EPO affirmed the inventiveness of upadacitinib – demonstrated that upadacitinib has an inhibitory effect on JAK1 and a selective inhibition of JAK1/3. However, the description of the subject patent failed to focus on other types of JAK activity and selectivity, apart from JAK3.
The CNIPA therefore concluded that the experimental results on JAK1 and JAK2 activity furnished by AbbVie and the selectivity deduced therefrom exceeded the teaching that a person skilled in the art could possibly obtain from the original patent filings. The aforesaid supplementary experimental evidence was found to be inadmissible.
Due to the lack of evidence to prove better technical effects of upadacitinib compared to the closest prior art, chemical formula Ic of Evidence 2, the CNIPA boiled down the technical problem solved by upadacitinib to the mere offering of a compound with a structure similar to that of the compound as disclosed by Evidence 2. Based on such findings, the CNIPA found that a person skilled in the art could obviously obtain upadacitinib, and the subject patent is devoid of inventiveness.
Comment
The CNIPA addressed in the invalidation decisions quite a few hotly debated issues concerning the examination of pharmaceutical compound patents – in particular, priority assessment and the admissibility of supplementary experimental data – which may provide some guidance on the drafting strategy and the building of a patent portfolio in China.
The CNIPA elucidates that from the perspective of a person skilled in the art, the supplementary experimental data serves as corroborative evidence of the effects that have been clearly and explicitly disclosed in the original filing documents, rather than a refocusing on the possible effects that are generally recorded in such documents.
In this case, although the potential inhibitory activity of upadacitinib on the JAK family is mentioned in the description of the subject patent, there are no experimental results on other types of JAK activity, apart from JAK3.
Should the supplementary experimental data be admitted solely based on the content mentioned in general, the inventor would list in the filing documents as many potential uses or effects as possible, without investing any substantial work or research. In that event, a patent could be granted based on the technical contribution made and published after the filing date of the patent. This would contravene the fundamental rule of the patent regime, where patent protections are offered in exchange for the public disclosure of new and useful inventions.
The case also offers practitioners a glimpse of the CNIPA’s examination criteria regarding supplementary experimental data. In assessing its admissibility, the agency has been enforcing a strict rule that only supplementary experimental data that serves as corroborative evidence of the effects that have been clearly and explicitly disclosed in the original filing documents would be admitted.
Patentees are also advised to take heed that insufficient disclosure during the drafting stage of the patent could backfire and jeopardise the stability of the granted patent.
There is evidence that the inventor had conducted JAK1 and JAK2 activity tests for upadacitinib and a series of other compounds before filing the subject patent application. However, the experimental results were not recorded in the description of the subject patent, probably for fear of divulging technical secrets. The omission led to the absence of necessary activity data to prove the effects of upadacitinib in the patent invalidity procedure, a flaw which is difficult to overcome by submitting supplementary experimental data.
Discovering a drug is often a lengthy and pricy process. Inventors attempting to protect their inventions from the outset could file multiple applications for their discoveries in different phases. The applications need to be carefully drafted so that they make sufficient disclosure of the new and incremental technical selection or improvement over the previous inventions, and maintain a certain level of overlap in terms of the scope of protection with the previous inventions. In such case, when an earlier patent expires, the core compounds would still fall within the protection scope of a later patent, which could markedly prolong the period of protection of the patentee’s core compounds.












