Patentability of human embryonic stem cells
The amended Patent Examination Guidelines, which China National Intellectual Property Administration (CNIPA) has been implementing since November 1 2019, have brought significant changes to the utilisation of human embryonic stem cells. The new guidelines allow technology relating to the stem cells isolated or procured from human embryos within 14 days after fertilisation but without developing in vivo, to be eligible subject matter, which means such technology will no longer be deemed non-patentable on the grounds of “contrary to social morality” as stipulated in Article 5 of the Patent Law.
The amended guidelines are in line with the Guiding Principles on Ethics of Human Embryonic Stem Researches, jointly issued by China’s Ministry of Science and Technology and the Ministry of Health on December 24 2003. These prohibit the research and utilisation of human embryonic stem cells for any reproductive purposes by providing that: 1) for utilising the blastula obtained from fertilisation in vitro, nuclear transplantation of somatic cells, parthenogenesis or genetic modification, its developing period in vitro should not exceed 14 days; 2) human blastula obtained for research purpose should not be implanted into the reproductive system of human beings or any other animals; and 3) germ cell of human beings should not be combined with that of any other species.
Human embryonic stem cell is a promising technology that may hold the keys to innovative treatment of various human diseases. However, due to ethical concerns, views are divided and jurisprudence varied as to the study and exploitation of human embryonic stem cells in different jurisdictions. Recognition of the patentability of human stem cell technology will fuel innovation and promote its commercialisation in the biomedicine field in China.
Analysis of a recent SPC exemplary case relating to biotechnology
In December 2019, the Supreme People’s Court (SPC) issued a judgment revoking the reexamination decision made by the Reexamination and Invalidation Department of the CNIPA (previously known as the Patent Reexamination Board, and hereinafter referred to as the CNIPA). The SPC expatiates on the application of different substantive requirements for granting a patent in this case. However, it is much more intriguing that it is one of the few cases where the SPC overturned the reexamination decisions made by the CNIPA in a patent administrative litigation, given that statistics in 2018 indicate that only 1.56% of the patent administrative suits challenging the CNIPA’s reexamination decisions are brought by applicants and among these cases, the first instance court upholds an astonishing 91.72% of CNIPA decisions (meaning only 8.28% decisions are rescinded).
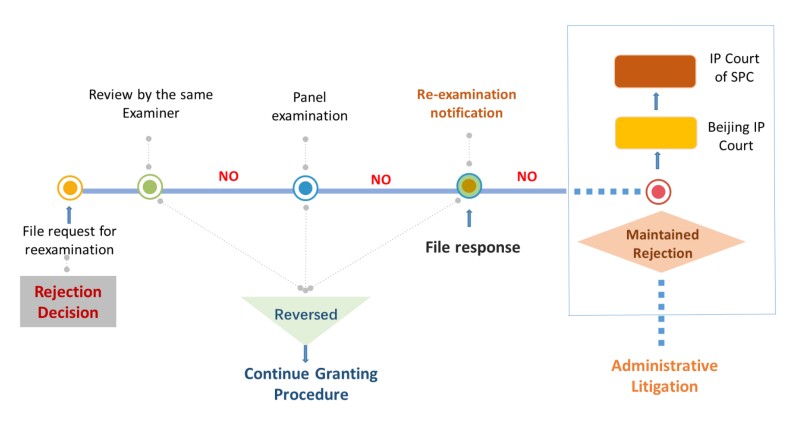
The above case relates to the patent application No. 20121005766.0 entitled “Binding Molecules” in the biotechnology field, specifically relating to a method for the production of a VH heavy chain-only antibody by immunising a transgenic rat expressing a heterologous VH heavy chain locus with an antigen. The applicants are Erasmus University Medical Centre Rotterdam and Mr Craig in the Netherlands. This application was subjected to reexamination before the CNIPA after final rejection due to lack of inventiveness, then to administrative litigation before the Beijing IP Court in the first instance and the SPC in the second instance, with the proceedings illustrated in the appendix. After the Beijing IP Court revoked the Reexamination Decision, the CNIPA appealed to the SPC but failed to reverse the judgment. The SPC judgment addresses the following issues.
Technical problem actually solved by the invention
In this case, the CNIPA and the courts utilised a substantially identical approach to assess inventiveness: 1) determining the closest prior art; 2) determining the distinguishing features of the invention and the technical problem actually solved by the invention; and 3) determining whether the claimed invention is obvious to a person skilled in the art. The main divergence lies in how to define the technical problem actually solved by the invention. The closest prior art cited is D1 (WO 02/085945A2) and one of the ascertained distinguishing feature is “the V gene segment is a naturally-occurring V gene segment derived from human”. Such a feature was incorporated into claim 1 from its dependent claim at the time of filing the reexamination request before the CNIPA. In the second instance, the CNIPA held that the technical effect of “naturally-occurring V gene segment derived from a human” was not demonstrated in the description of the patent application. Thus, the technical problem actually solved would not include such a feature. The SPC rebutted in two aspects: 1) the CNIPA redefined, in the appeal request, the technical problem solved, as compared with that in the reexamination decision, and 2) such an issue would fall into the scope of insufficient disclosure and should not be mixed up with the inventiveness for assessment.
The boundary for different parameters for granting a patent and the order of examination
In this case, the dispute focused on whether the claimed invention is inventive. The SPC leveraged the opportunity to elucidate the role of different parameters for granting a patent. The SPC held that the parameters of inventiveness, sufficient disclosure and claim support play different roles in ascertaining patentability and need to apply independently. As for the order of examination, in principle, sufficient disclosure, claim support and amendments beyond the original disclosure shall precede novelty and inventiveness. Otherwise, the examination of novelty and inventiveness would be built on a shaky foundation, which would make the procedure uneconomical.
As mentioned above, the key feature of “the V gene segment is a naturally-occurring V gene segment derived from human” was incorporated into claim 1 from its dependent claim at the time of filing the reexamination request before the CNIPA. As in practice the scope of reexamination is generally limited to the contents of the rejection decision, the CNIPA opined that the aforesaid feature is obvious over D1 and there is no proof of the corresponding technical effect of “producing a functional human VH heavy chain-only antibody” in the description. It is possible that for the sake of examination efficiency, the CNIPA, when making the reexamination decision, focused on the inventiveness findings of the rejection decision, considering that the sufficiency requirement might be easier to reach.
“Hindsight” in the assessment of inventiveness
To avoid hindsight, the SPC opined that the motivation derived from the prior art should be concrete and definite technical solution(s), rather than research direction or needs in general. This could insinuate the importance of evidence in evaluation of technical motivation of the claimed invention from the prior art. In fact, the amended Patent Examination Guidelines implemented on November 1 2019 added the requirement of objective evidence in relation to common knowledge during the examination procedure. This requires the examiner to produce evidence where the applicant opposes the use of common knowledge or the technical feature contributing to technical solution is objected to as common knowledge. Both the SPC and CNIPA has been emphasising the role of prior art as evidence, including common knowledge.
Submission of new evidence during administrative litigation proceedings
The SPC found the seven pieces of supplementary evidence adduced by the applicants before the Beijing IP Court admissible. The SPC held that although the evidence was not submitted during the reexamination stage, considering its authenticity, legality and relevance, refusal of such corroborative evidence, which may help understand the state of the art, would deprive the applicants of the opportunity for any remedies. Based on the description of this patent application and in view of such evidence, the SPC concluded that it is not obvious over D1 by using “a naturally-occurring V gene segment derived from human” to substitute “a camelid V gene segment” in the claimed technical solution to obtain a human VH heavy chain-only antibody of better water solubility. It seems that the SPC would allow a certain amount of leeway in supplementing evidence in the judicial proceeding. The case is listed in the 36 exemplary technology-related IPR cases, which the SPC’s Intellectual Property Court concluded in 2019.
Appendix
Re-examination Proceeding before CNIPA and Administrative Litigation