
A lack of accessible prior art in the cannabis industry is leading to overly broad or weak patents passing examination at the USPTO that may block innovation, according to medical marijuana companies.
In-house sources say that some companies and law firms have filed overly broad, obvious or non-novel claims, betting that they will likely pass scrutiny because examiners are not yet adequately trained in the field and do not have access to prior art examples to compare applications against.
This situation has emerged because of the relative infancy of the US legal cannabis industry and the fact that few academic articles or other forms of technical research have been published on cannabinoid development.
“I don’t know if these weak patents are inhibiting innovation yet, but they will if people believe they can be enforced,” says Mowgli Holmes, chief scientific officer at Phylos Bioscience. “There is no question that the patents being granted now radiate a lack of experience on the part of the USPTO.”
He adds that most budding industries have had to put up with similar situations at patent offices in the past until more specialist examiners were hired, employees were better trained and prior art documents were drawn up and made more available.
“The USPTO is missing some prior art and clearly issued a few patents for inventions that prior art already exists for,” says Kevin McKernan, chief scientific officer at Medicinal Genomics. “We’re not necessarily affected by them but we were recently funded to sequence a cannabis genome that may challenge those weak patents.”
Marijuana was illegal across the US until California became the first state to legalise the substance for medical use in 1996. Cannabis was first legalised for recreational use on a state level in 2012 but is still deemed illegal for all uses by the federal government. The drug is now legal for medical use in 33 states and 10 of those have also legalised it for recreational use.
The legalisation on cannabis led to a large number of cannabis patent filings in the US. According to Clarivate Analytics, more than 1,500 cannabis-related patents have been filed at the USPTO since 2005.
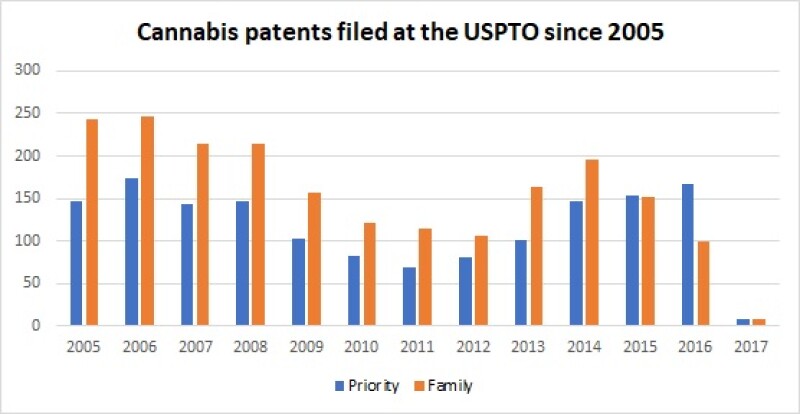
Cannabis patents filed at the USPTO since 2005 |
||||||||||||||
2005 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
Total |
|
Priority |
147 |
174 |
144 |
146 |
102 |
82 |
68 |
80 |
101 |
146 |
153 |
167 |
8 |
1518 |
Family |
243 |
247 |
214 |
214 |
156 |
122 |
114 |
106 |
163 |
195 |
152 |
100 |
8 |
2034 |
Source: Clarivate Analytics |
||||||||||||||
Weeding bad patents
Despite the medical cannabis sector first emerging in the US in the 1990s, a patent war to invalidate weak marijuana registrations may have only just started.

|
|||
|
The first infringement case on a cannabis-related product, United Cannabis Corp (UCANN) v Pure Hemp Collective, was filed in August 2018. It remains to be seen whether UCANN’s reportedly overly broad patent for “cannabis extracts and methods of preparing and using same” (US patent number 9,730,911) will be held valid and enforceable – or whether the case will be thrown out altogether.
Depending on the outcome of this case, many other cannabis patents at the USPTO may soon face invalidation challenges. Reggie Gaudino, vice-president of IP at Steep Hill Labs, says one good example of an unfairly inhibiting patent he came across was for a large number of cannabis strains hidden in a method patent. The patent covers a natural process, long known in the cannabis industry, for strains containing 3% THC, 3% CBD and at last 1% terpene.
He says that another example is a patent for the formulation of CBD medicines that claims formulations with very high percentages of the cannabinoid. But people have long known how to purify CBS to the point where it becomes crystalline, he says, which is already between 88% and 99% pure.
“The PTO had no reference that these techniques have been known for a long time, so these patents were issued,” he explains. “[The first example] now has the potential to disrupt half of the medical cannabis market, which is all about the combination of THC and CBD, because those strains have been taken away.
“Yet the people that have that patent did not breed every related strain in existence – they bred one strain and went after all of them.”
Holmes at Phylos adds that the industry is facing a biopiracy situation where a company might come to the USPTO with a technique or strain that has existed for years. And because the invention was developed in the cannabis underground, perhaps in another country, and out of sight of the modern legislative apparatus, the patent office would not know that it was not novel.
Gaudino points out that there is also the potential for companies to accidentally infringe on patents at the USPTO because of the lack of prior art and the way cannabis strains are developed. He explains that growing conditions can be manipulated to develop similar chemical profiles on genetically unrelated strains.
“When you add the genetic component you must also add an environmental one – but to get quality patents now, they would require far more detailed information to be disclosed, and the patent office cannot require that.”
Path to higher learning
Sources say that examiner knowledge of marijuana cultivation will develop over time, as it did with other emerging industries such as software. But encouraging the development of new prior art may be trickier than it was for other sectors.
Gaudino points out that generating and publishing new research on cannabis is a risk for organisations because the substance is still illegal federally. He says that few firms are willing to conduct or publish the necessary research for fear of incurring the wrath of the US Drug Enforcement Administration (DEA).
“There are very few companies in the US willing to do that research because of the risks involved,” he says.

|
|||
|
“Universities and doctors cannot do it and there are a handful of labs like mine that fly in the face of the DEA and do the research and try to publish it knowing full well the administration could fly down one day and stomp all over us.”
McKernan at federally adds that this fear was more of a pressing problem before 2013 when the Cole Memorandum was issued. He says that a lot of the reluctance for companies to tie their name to particular a genotype or strain has gone away and more are starting to put their names on state-run cannabis grows.
The solution to the problem, of course, is federal legalisation – which may be closer than some companies might think. Dale Hunt, legal adviser for the Open Cannabis Project, believes federal legalisation is likely in the next three years because of the financial and moral momentum medical marijuana – and, to a lesser extent, recreational cannabis – is gaining.
There is also the challenge of making cannabis innovations that have existed for hundreds or thousands of years but are often held in non-traditional sources readily available to USPTO examiners.
Cannabis organisations such as the Open Cannabis Project are attempting to create a database of this prior art and emerging documents to ensure patent examiners have access to all documents they need to make the right decisions in cannabis examinations.
Holmes says: “The cannabis industry has not emerged out of nowhere, it has just come into the light. People out there were doing things with cannabis for a long time but they had no way to document them because of federal illegality.” He adds that projects such as the Open Cannabis Project are to find and publish the missing information.
Gaudino at Steep Hill Labs points out that ancient Chinese societies knew CBD could be used to treat inflammation. And, as late as 1890, Queen Victoria’s physician J Russel Reynolds wrote in the Lancet that: “In all painful maladies I have found Indian hemp by far the most useful of drugs. It is especially so in those cases which are, to the present time, relegated to the ‘functional’ order. Neuralgia, periodic or not, has often yielded to cannabis indica-pure.”
Gaudino adds that there are a few cases made where there is so much knowledge in the industry and forums can be used, so long as the body of data is large enough.
Medicinal Genomics also publishes its data on a sister repository website called Kannapedia, which publishes most of the cannabis and hemp strains that have been genetically sequenced by the company. The site uses blockchain to record and provide proof of time stamps for particular strains.
“Making the sequencing data public is very important – you need to have data sequencing of cannabis genotypes and phenotypes made available for the USPTO to be able to reproduce innovations,” says McKernan at Medicinal Genomics.
He adds that it is also important to store the information in something such as blockchain that is decentralised and cannot be easily taken down by interested parties.
Scientists are only beginning to tap the potential of cannabis compounds in the medical world. But if that innovation is to continue, real change needs to be made across US institutions and marijuana companies must help with the prior art documentation project where they can.











